
Experiment 4 - Properties of ethanol
Assessment
•
Pan Wai Seng
•
Chemistry
•
University
•
57 plays
•
Medium
Student preview

10 questions
Show answers
1.
Multiple Choice

Why is the pH of distilled water acidic?
test tube contain impurities
absorption of atmospheric carbon dioxide into the distilled water
Distilled water is naturally acidic
2.
Multiple Choice
Ethanol dissolves in water because it forms ______ bonds with water molecules.
dative
hydrogen
metallic
covalent
3.
Multiple Choice
Select the correct structure of triiodomethane.
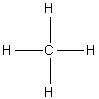
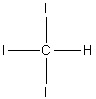
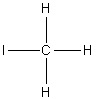
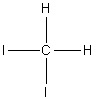
4.
Multiple Choice

Gas bubbles produced when sodium is placed into ethanol because sodium displace ______ from ethanol.
oxygen
nitrogen
carbon
hydrogen
5.
Multiple Choice

Why is sodium metal kept in paraffin oil?
To reduce the contact of sodium metal with vapour in the air
To prevent the sodium metal from decomposing
To keep the sodium metal fresh
To maintain the glossiness of sodium metal
Explore all questions with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Esters, Carboxylic Acids, and Amides!
•
University

Organic chemistry II
•
University

Pharmaceutical Chemistry
•
University

Organic Compounds with Hydrogen
•
University

ORGANIC CHEMISTRY
•
10th Grade - University

Esters
•
University

3.3.9.1 Carboxylic acids and esters (A-level only)
•
10th Grade - University

3.3.9 Carboxylic acids + derivatives (A-level only)
•
10th Grade - University