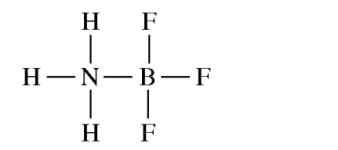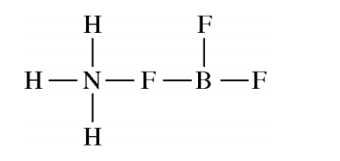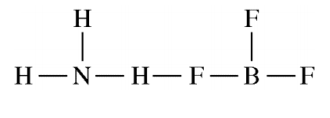Bonding and Intermolecular Forces Review Extended
Assessment
•
Rondel Thorpe
•
Science, Physics, Chemistry
•
9th Grade - University
•
13 plays
•
Hard
Student preview

36 questions
Show all answers
1.
MULTIPLE CHOICE
30 sec • 1 pt

Which of the following can be inferred from the diagram above that shows the dependence of potential energy on the internuclear distance between two atoms?
Answer explanation
The minimum potential energy occurs at an internuclear distance of 75pm, which corresponds to the length of the stable bond that forms between the two atoms. If the atoms were any closer to each other, the net force would be repulsive. Likewise, if the atoms were farther from each other, the net force would be attractive. At 75pm, the net force between the two atoms is zero.
2.
MULTIPLE CHOICE
30 sec • 1 pt

Based on the data in the tables above, which of the following statements provides the best prediction for the boiling point of NaCl ?
Answer explanation
The Cl− ion is larger than the F− ion so the attractive interactions in NaCl are weaker than in NaF.
3.
MULTIPLE CHOICE
30 sec • 1 pt
Has a central atom with less than an octet of electrons
4.
MULTIPLE CHOICE
30 sec • 1 pt
Has two lone pairs on the central atom.
5.
MULTIPLE CHOICE
30 sec • 1 pt
Predicted to have the largest bond angles.
6.
MULTIPLE CHOICE
30 sec • 1 pt
Has trigonal pyramidal molecular geomtry.
7.
MULTIPLE CHOICE
30 sec • 1 pt

The potential energy as a function of internuclear distance for three diatomic molecules, X2, Y2, and Z2, is shown in the graph above. Based on the data in the graph, which of the following correctly identifies the diatomic molecules, X2, Y2, and Z2?
8.
MULTIPLE CHOICE
30 sec • 1 pt
The lattice energy of a salt is related to the energy required to separate the ions. For which of the following pairs of ions is the energy that is required to separate the ions largest? (Assume that the distance between the ions in each pair is equal to the sum of the ionic radii.)
Answer explanation
The most tightly bonded ions will be those with the highest oxidation number. Though Mg and Ca both have an oxidation number of 2+ , since Mg is the smaller ion (atom as well), it will have a smaller radius leading to a stronger bond and higher lattice energy.
9.
MULTIPLE CHOICE
30 sec • 1 pt
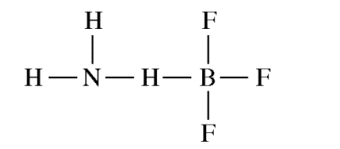
NH3 reacts with BF3 to form a single species. Which of the following structural diagrams is the most likely representation of the product of the reaction?
Answer explanation
The lone pair of N can be shared and both N and B will have an octet.
F and H can only form one bond and will not be able to bond twice as shown in the incorrect options.
10.
MULTIPLE CHOICE
30 sec • 1 pt
The melting point of MgO is higher than that of NaF. Explanations for this observation include which of the following?
I. Mg2+ is more positively charged than Na+.
II. O2- is more negatively charged than F-.
III. The O2- ion is smaller than the F- ion.
Explore all questions with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)