
Quantum Mechanical Model
Assessment
•
Breanna Malmos
•
Science
•
9th - 12th Grade
•
71 plays
•
Medium
Student preview

10 questions
Show answers
1.
Multiple Select
There are four different orbital shapes. Select them from the following list.
"s"
"f"
"g"
"d"
"p"
2.
Multiple Choice
Which shape represents the shape of an "s" orbital?
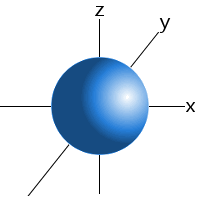
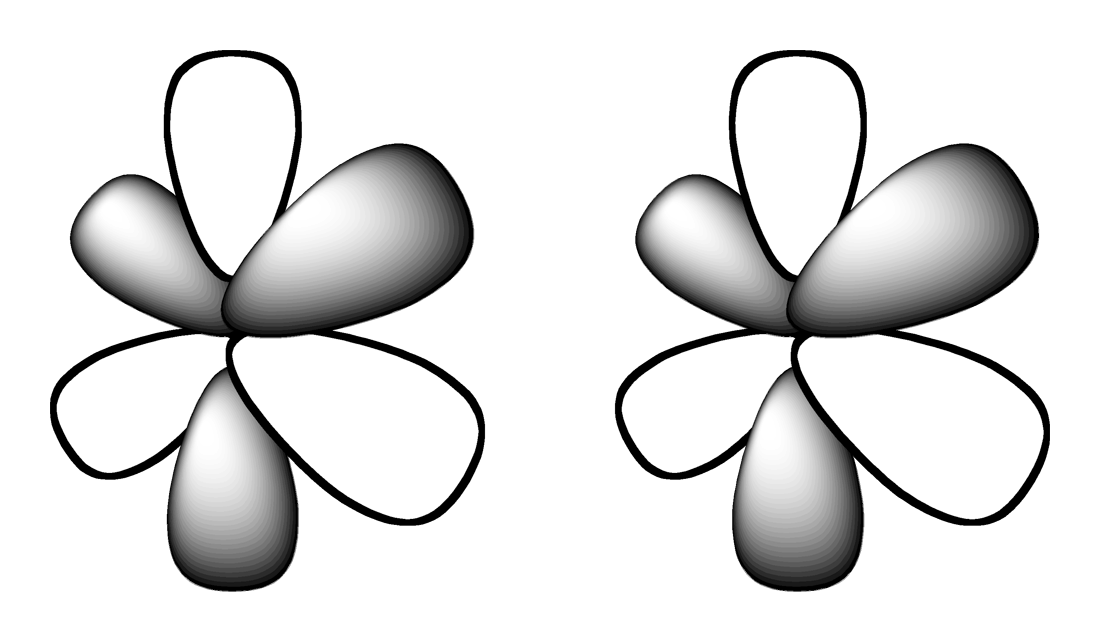
3.
Multiple Choice
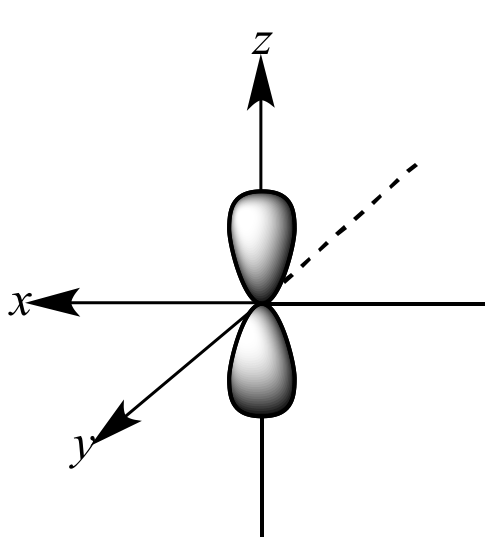
Which shape represents the shape of an "p" orbital?



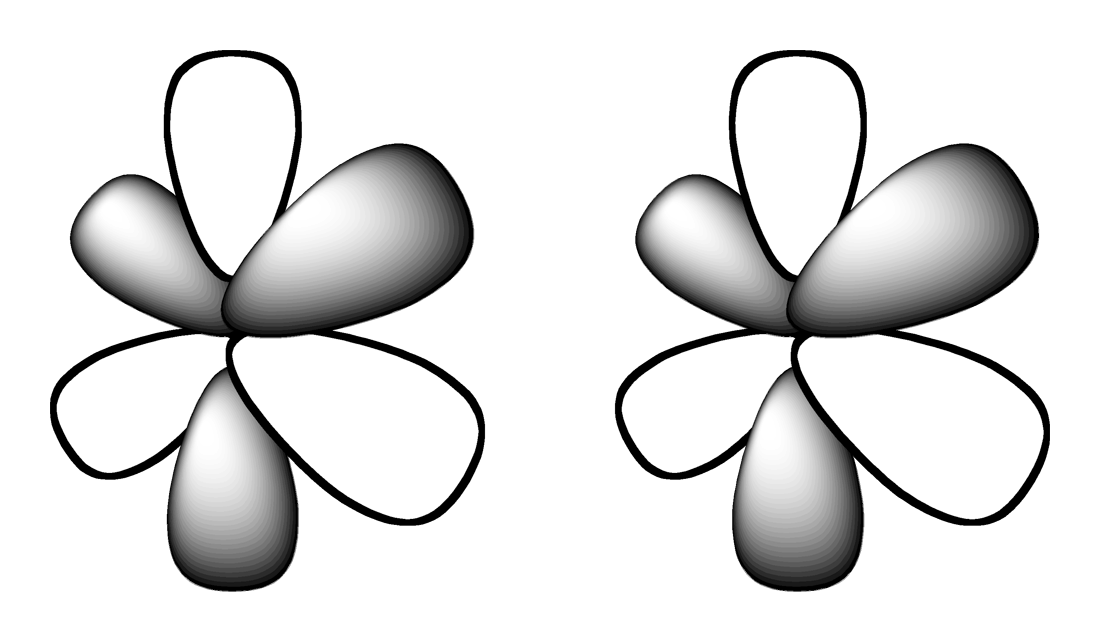
4.
Multiple Choice
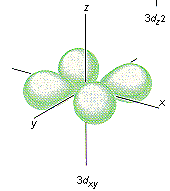
Which shape represents the shape of an "d" orbital?



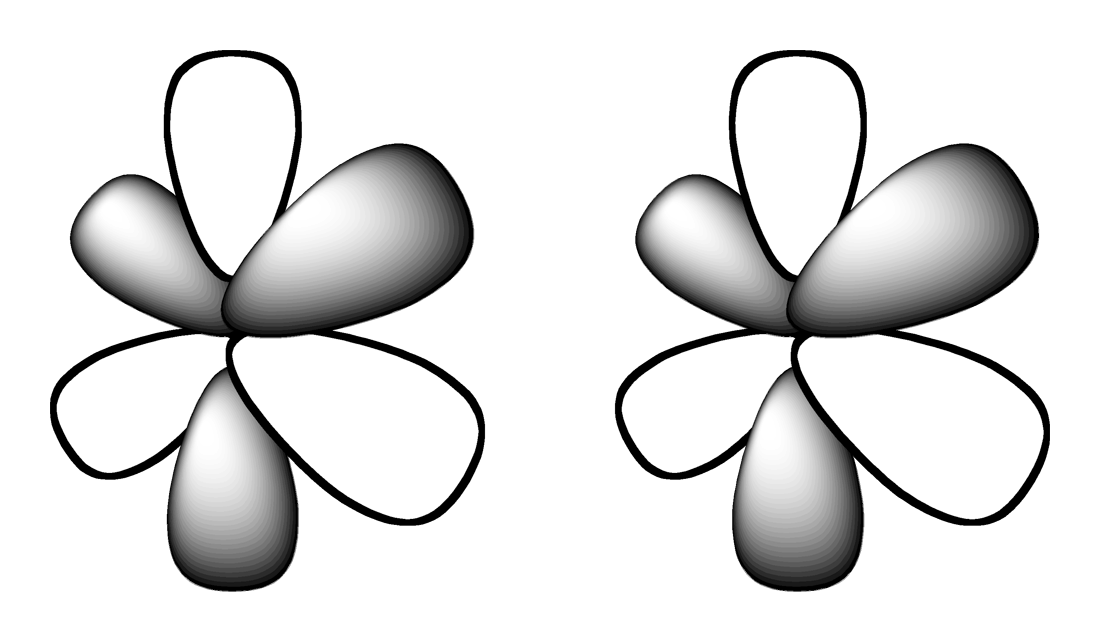
5.
Fill in the Blank
How many different types of "s" orbitals are there?
Explore all questions with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Quantum mechanical model
•
9th Grade

Quantum Mechanical Model of the Atom
•
9th - 12th Grade

Quantum Model
•
9th Grade - University

Schrodinger Orbital
•
9th - 12th Grade

Quantum Mechanical Model of the Atom
•
9th Grade - University

QUANTUM NUMBERS
•
9th Grade

Quantum Mechanical Model of an Atom
•
9th Grade

Quantum Model
•
9th Grade - University