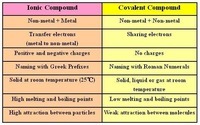
Naming Ionic and Covalent Compounds
Assessment
•
Sara Hodžić
•
Other Sciences
•
KG - University
•
1 plays
•
Easy
Student preview

15 questions
Show answers
1.
Multiple Choice
calcium chlorine.
calcium chlorite.
calcium chloride.
chlorine calcium.
2.
Multiple Choice
sulfur dioxide.
sulfur oxide.
disulfur oxide.
sulfide oxygen.
3.
Multiple Choice
copper oxygen.
copper oxide.
dicopper oxide.
copper(II) oxide.
4.
Multiple Choice
nitrogen oxide.
dinitrogen oxide.
nitrogen pentoxide.
dinitrogen pentoxide.
5.
Multiple Choice
2+
1+
0
1-
Explore all questions with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Naming and Writing Ionic and Covalent Compounds
•
KG - University

Ionic/Covalent Compounds
•
KG - University

Naming Ionic and Covalent Compounds
•
KG - University

Naming Ionic and Covalent Compounds
•
KG - University

Naming Ionic and Covalent Compounds
•
KG - University

Naming Ionic and Covalent Compounds
•
KG - University

Naming and Forming Ionic and Covalent Compounds
•
KG - University

Naming and Writing Ionic and Covalent Compounds
•
KG - University