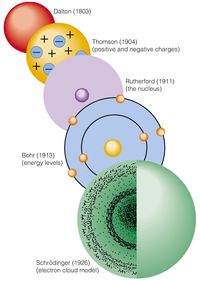
History of Atomic Models
Assessment
•
Sakinah Ellickson
•
Chemistry
•
9th Grade
•
451 plays
•
Medium
Student preview

15 questions
Show all answers
1.
MULTIPLE CHOICE
45 sec • 1 pt

Whose model suggested that negative particles were mixed in with positively charged material - like seeds in a watermelon?
2.
MULTIPLE CHOICE
45 sec • 1 pt
Who discovered that the atom had an small, dense, positively charged center?
3.
MULTIPLE CHOICE
1 min • 1 pt
Place the following atomic models in order, from earliest to latest:
A) Rutherford B) Thomson C) Dalton
4.
MULTIPLE CHOICE
45 sec • 1 pt
5.
MULTIPLE CHOICE
45 sec • 1 pt
Who named the positive center of the atom the "nucleus?"
6.
MULTIPLE SELECT
45 sec • 1 pt
Which scientists believed an atom was indivisible? (Choose all correct responses.)
7.
MULTIPLE CHOICE
45 sec • 1 pt
Which scientist discovered that the atom has parts - which means it can be divided?
8.
MULTIPLE CHOICE
45 sec • 1 pt
Which scientist first suggested that atoms could join?
9.
MULTIPLE CHOICE
45 sec • 1 pt
Which scientist believed that elements differed from one another because their atoms had different mass?
10.
MULTIPLE CHOICE
1 min • 1 pt

In the atomic model nicknamed the "plum pudding" model, what do the plums represent?
Explore all questions with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)