Valence Bond Theory (VBT) & Hybridisation
Assessment
•
Ekta Vishnoi
•
Chemistry
•
12th Grade
•
8 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
11 questions
Show answers
1.
Multiple Choice
s-s overlap is a _______
2.
Multiple Choice
A π (pi) bond is the result of the
overlap of two s orbitals
overlap of an s orbital and a p orbital
overlap of two p orbitals along their axes
sidewise overlap of two parallel p orbitals
sidewise overlap of two s orbitals
3.
Multiple Choice
Sigma bonds are formed by
side to side overlap of s orbitals
end to end overlap of s orbitals
side to side overlap of p orbitals
end to end overlap of p orbitals
4.
Multiple Choice
Greater the overlap of the orbitals, _________________________
lesser the bond strength
greater the bond strength
bond strength remains the same
no bond formation
5.
Multiple Choice
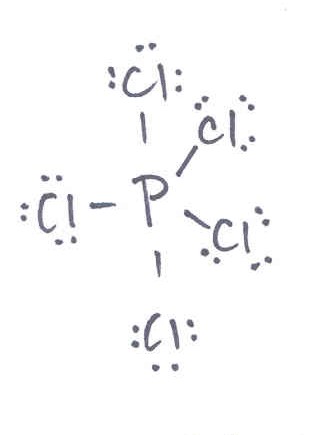
3 sigma and 2 pi
5 sigma and 5 pi
5 sigma and 0 pi
0 sigma and 5 pi
6.
Multiple Choice
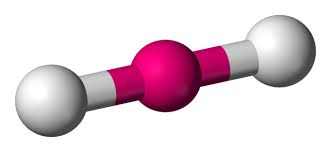
What is the hybridization of a linear molecule?
sp
sp2
sp3
sp3d

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Types of Bonding
•
10th - 11th Grade

Covalent Bonds
•
9th - 11th Grade

Covalent Bonding
•
10th - 12th Grade

Chemical Bonding
•
10th Grade

Stogs Naming Haloalkanes
•
11th - 12th Grade

Covalent Bonding
•
8th - 10th Grade

Covalent Bonding
•
9th Grade

Periodic Table
•
8th Grade