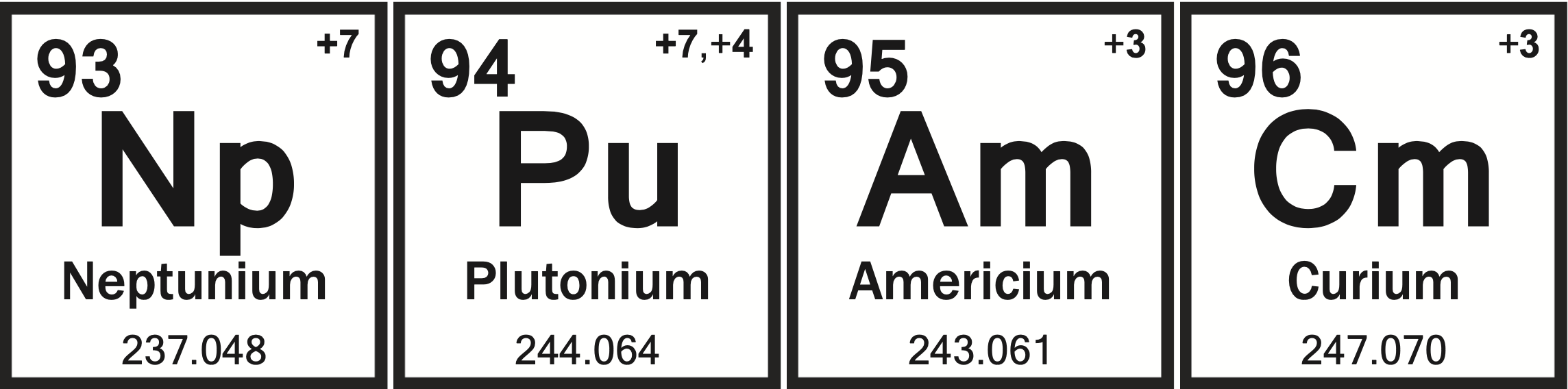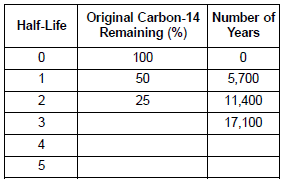
Nuclear Chemistry Quiz
Assessment
•
Robert RolesvilleHS
•
Science
•
10th - 12th Grade
•
120 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
12 questions
Show answers
1.
Multiple Choice
What is the correct # of protons, electrons, and neutrons for Cobalt - 59?
27 protons, 59 electrons, & 27 neutrons
59 protons, 59 electrons, & 86 neutrons
27 protons, 27 electrons, & 32 neutrons
27 protons, 32 electrons, & 27 neutrons
2.
Multiple Choice
Which subatomic particle(s) determine the mass of the atom?
protons & neutrons
protons only
protons & electrons
neutrons & electrons
3.
Multiple Choice

What is the name of this isotope?
Germanium-32
Germanium-72
Germanium-72.64
Hafnium-72
4.
Multiple Choice
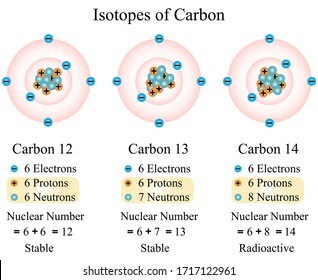
What is an isotope?
Atoms of the same element with different masses.
Atoms of the same element with different # of protons.
Atoms of the same element with different atomic numbers.
Atoms of the same element with the different # of electrons.
5.
Multiple Choice

What type of radioactive decay is pictured here?
Alpha decay
Beta decay
Gamma decay
Positron decay
6.
Multiple Choice
146C --> 0-1e + ________
145B
146C
147N
42He

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Atoms and The Periodic Table
•
8th - 10th Grade

Atoms Quiz
•
6th Grade

Atoms and Molecules
•
6th - 8th Grade

Properties of Elements
•
6th - 8th Grade

Periodic Table
•
9th Grade

Atoms
•
5th Grade

Counting Atoms
•
11th - 12th Grade

Atomic Structure
•
8th Grade