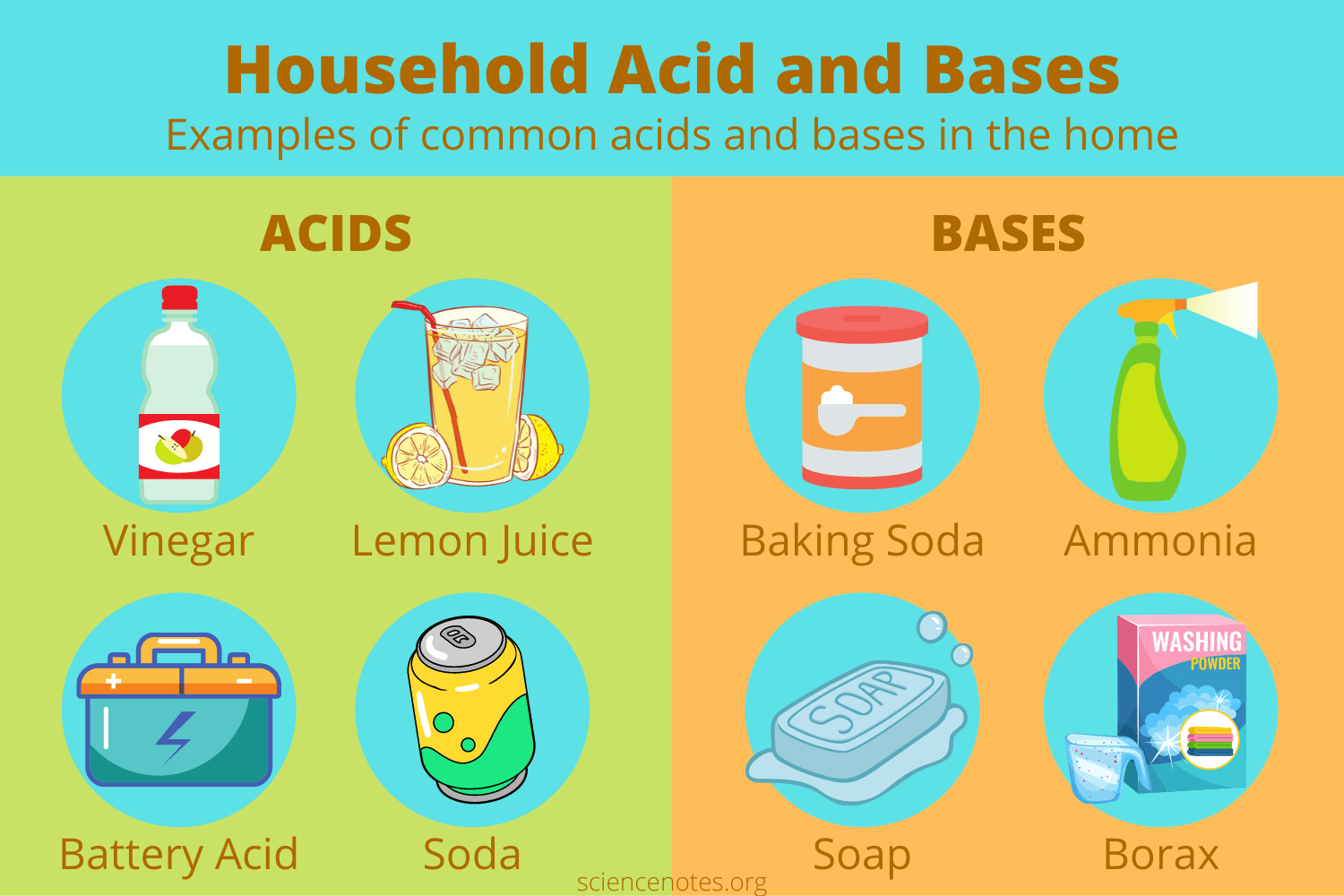
Acids and Bases
Assessment
•
Johnathan Hanrahan
•
Chemistry, Science
•
9th - 12th Grade
•
343 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
20 questions
Show answers
1.
Multiple Choice
A solution has a pH of 10.34
Is it an Acid or a Base?
Acid
Base
2.
Multiple Choice
A solution has a pH of 10.34
Is it an Acid or a Base?
Acid
Base
3.
Multiple Choice
What is the pH of a solution with an [H+] concentration of 1x10-7?
2.53
5.34
7.0
11.4
4.
Multiple Choice
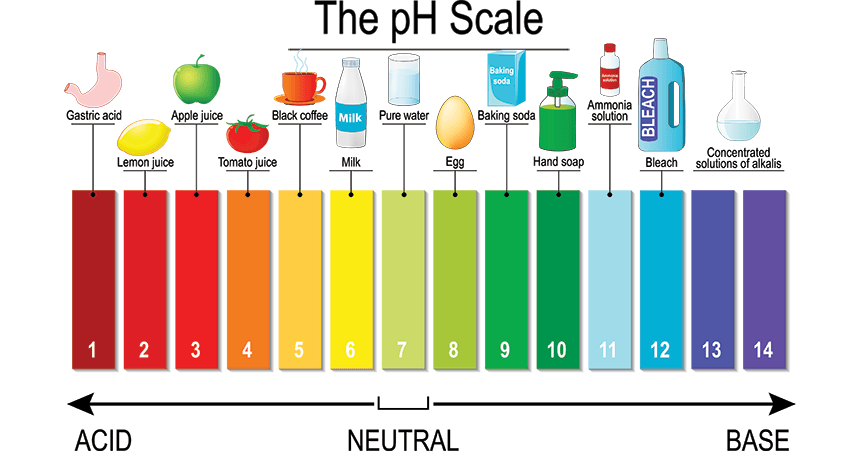
Which of the following represents an Acid?
HINT: Try calculating the pH!
pH = 12
[H+] = 5.4 x 10-4
pH = 8
[H+] = 2.3 x 10-10
5.
Multiple Choice
Which of the following represents an Acid?
HINT: Try calculating the pH!
pH = 12
[H+] = 7.9 x 10-8
pH = 2
[H+] = 2.3 x 10-10
6.
Multiple Choice

_____ is an expression of the concentration of Hydrogen Ions in a solution.
pH
pOH

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Naming Acids Practice
•
10th - 12th Grade

Molarity
•
10th - 12th Grade

Acids and Bases
•
8th Grade

Acids Review
•
10th Grade

Molarity & Dilution
•
11th - 12th Grade

Naming Acids and Bases
•
9th - 12th Grade

Acids and Bases
•
9th Grade

Titration
•
10th - 12th Grade