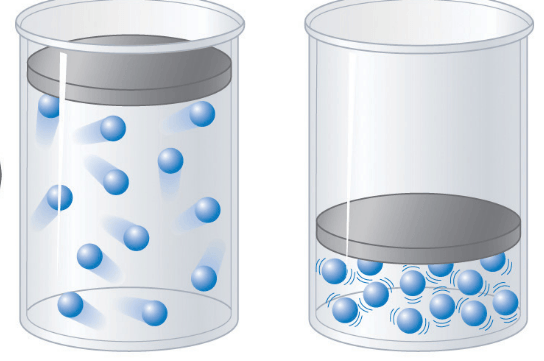
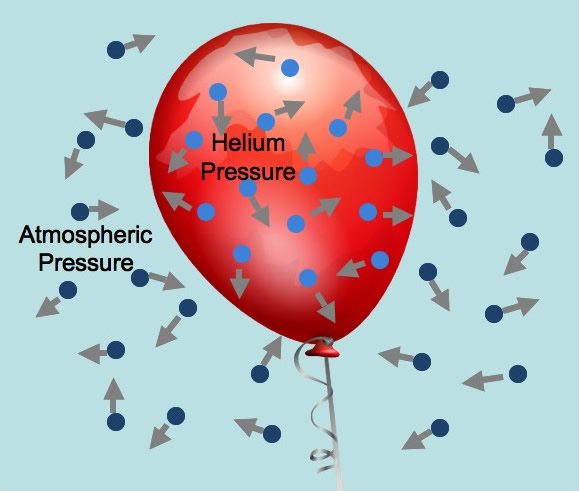
Gas Laws
Assessment
•
LAURENDA GAUTHIER
•
Chemistry
•
10th Grade
•
984 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
19 questions
Show answers
1.
Multiple Choice
speed up and move closer
speed up and move farther apart
slow down and move closer together
slow down and move farther apart
2.
Multiple Choice
in an orderly fashion
constantly and randomly
in straight line paths
in a circular motion
3.
Multiple Choice
True
False
4.
Multiple Choice
Increase
decrease
5.
Multiple Choice
Increase
Decrease
6.
Multiple Choice
direct
inverse
no relationship
I don't know

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Ideal Gas Laws
•
10th Grade - University

Phase Changes
•
10th - 12th Grade

States of Matter
•
9th Grade

States of Matter
•
10th Grade

Kinetic Molecular Theory
•
10th Grade - University

Phases of Matter
•
6th - 8th Grade

Vapor Pressure and Phase Diagrams Practice
•
10th - 11th Grade

States of Matter
•
9th - 10th Grade