
Extraction of Iron
Assessment
•
Rasyidah Rahim
•
Chemistry
•
10th - 11th Grade
•
141 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
8 questions
Show answers
1.
Multiple Choice
What is the name of the Iron ore?
Iron Chloride
Bauxite
Hematite
Iron Pyrite
2.
Multiple Choice
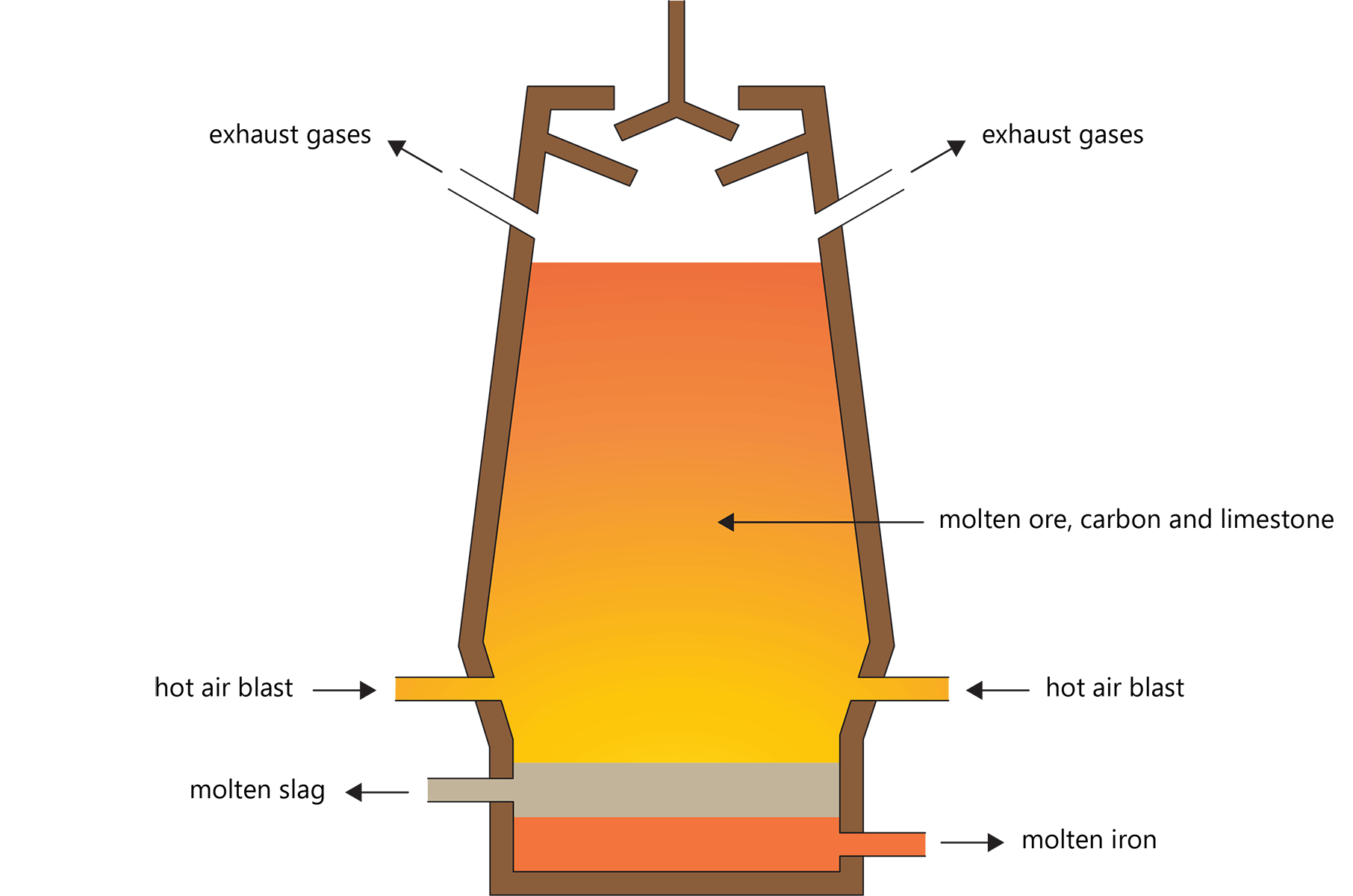
The diagram shows the blast furnace used in the extraction of Iron. The furnace is loaded with the 'Charge' which consists of
Calcium Oxide, Coke and Iron Ore
Calcium Carbonate, Coke and Iron Chloride
Calcium Carbonate, Coke and Iron Ore
Calcium Oxide, Coke and Iron Ore
3.
Multiple Choice
One of the major impurities in the iron ore is....
Silica, SiO2
Sulfur, S
Calcium Silicate, CaSiO3
Carbon
4.
Multiple Choice
The iron produced from the extraction process flows to the bottom of the furnace and is then tapped off because....
The temperature of the furnace is lower than the melting point of iron
The temperature of the furnace is higher than the melting point of iron
5.
Multiple Choice
The impurities in the iron ore will be reacted with Calcium Oxide to produce...
Sulfur dioxide
Calcium Silicate
Calcium Carbonate
Silica
6.
Multiple Choice
Calcium Carbonate (Limestone) is converted to Calcium oxide through which reaction
Nautralisation
Displacement
Thermal Decomposition
Exothermic

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Oxidation and Reduction
•
10th Grade

Electrolytic Cell
•
12th Grade

Mole Concept
•
University

Extracting Aluminium
•
11th Grade

Chemical Reaction and Equation
•
10th Grade

Element Symbols and Names
•
8th - 12th Grade

Electrolysis
•
7th Grade

Group Assignment
•
University