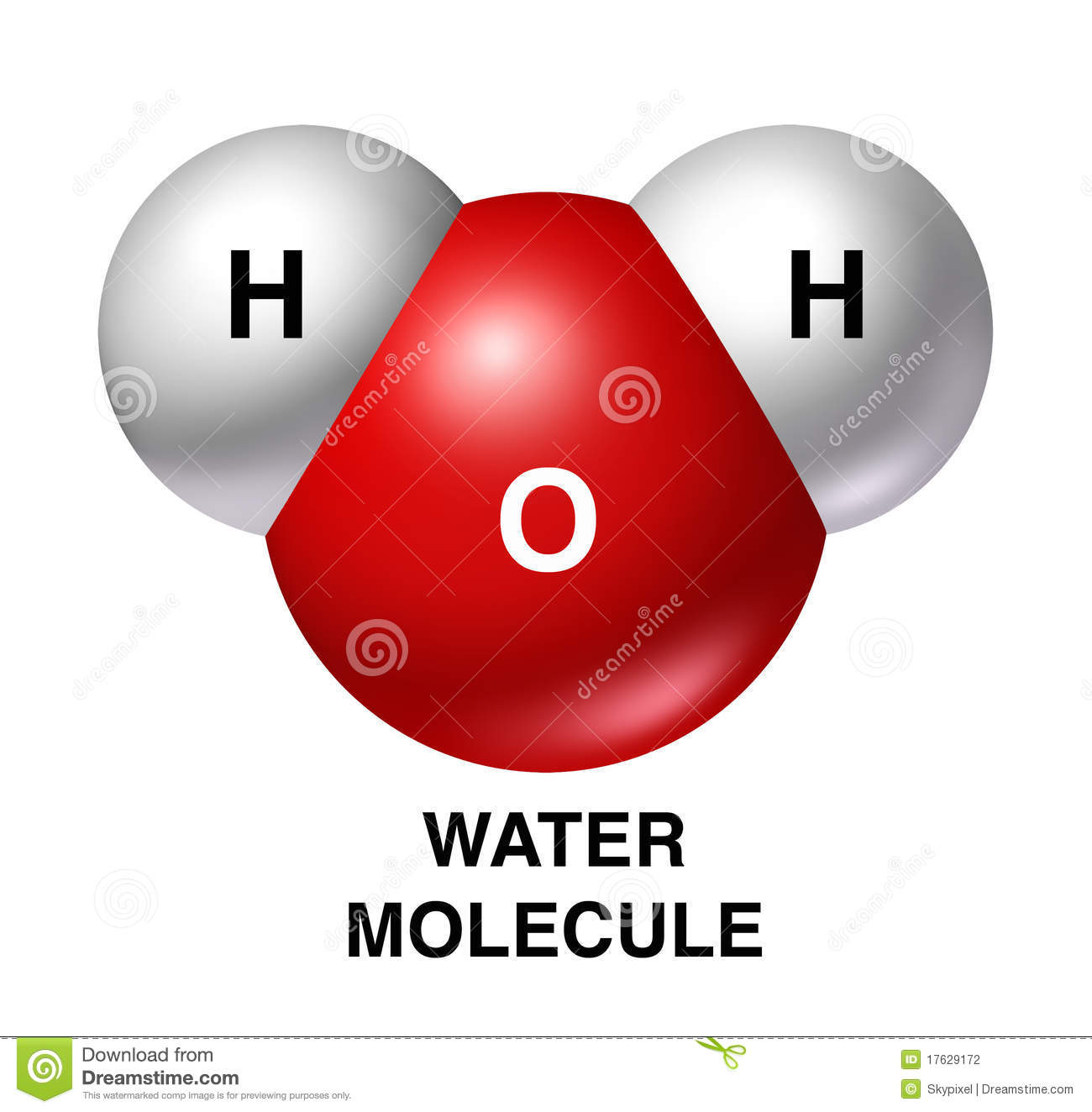
Water Structure and Properties
Assessment
•
Brandon Funderburg
•
Science
•
10th - 12th Grade
•
33 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
59 questions
Show answers
1.
Multiple Choice

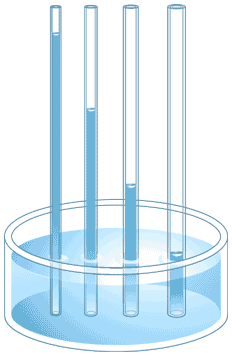
cohesion
adhesion
capillary action
surface tension
2.
Multiple Choice
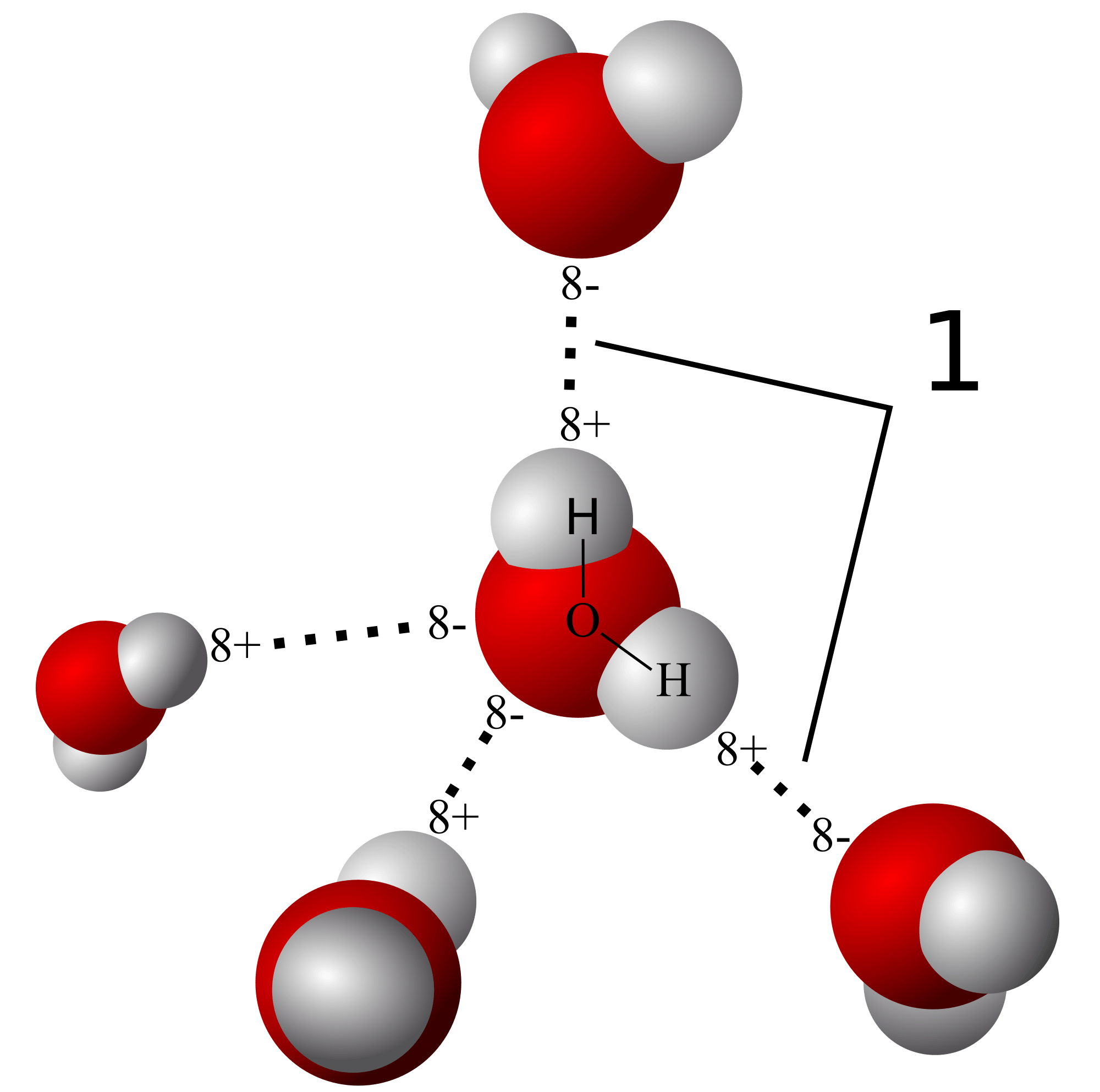
Covalent bonds
Ionic bonds
Polar bonds
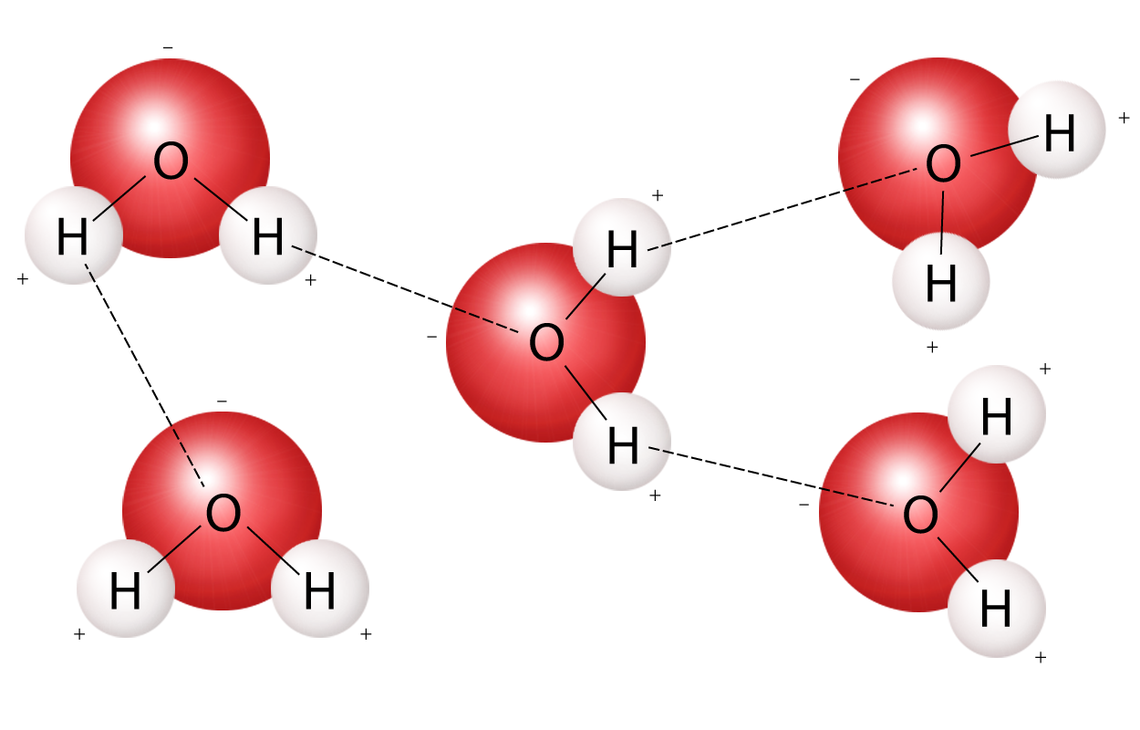
Hydrogen bonds
3.
Multiple Choice
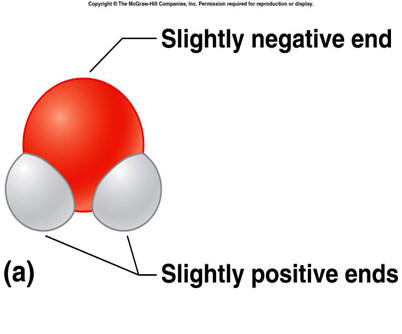
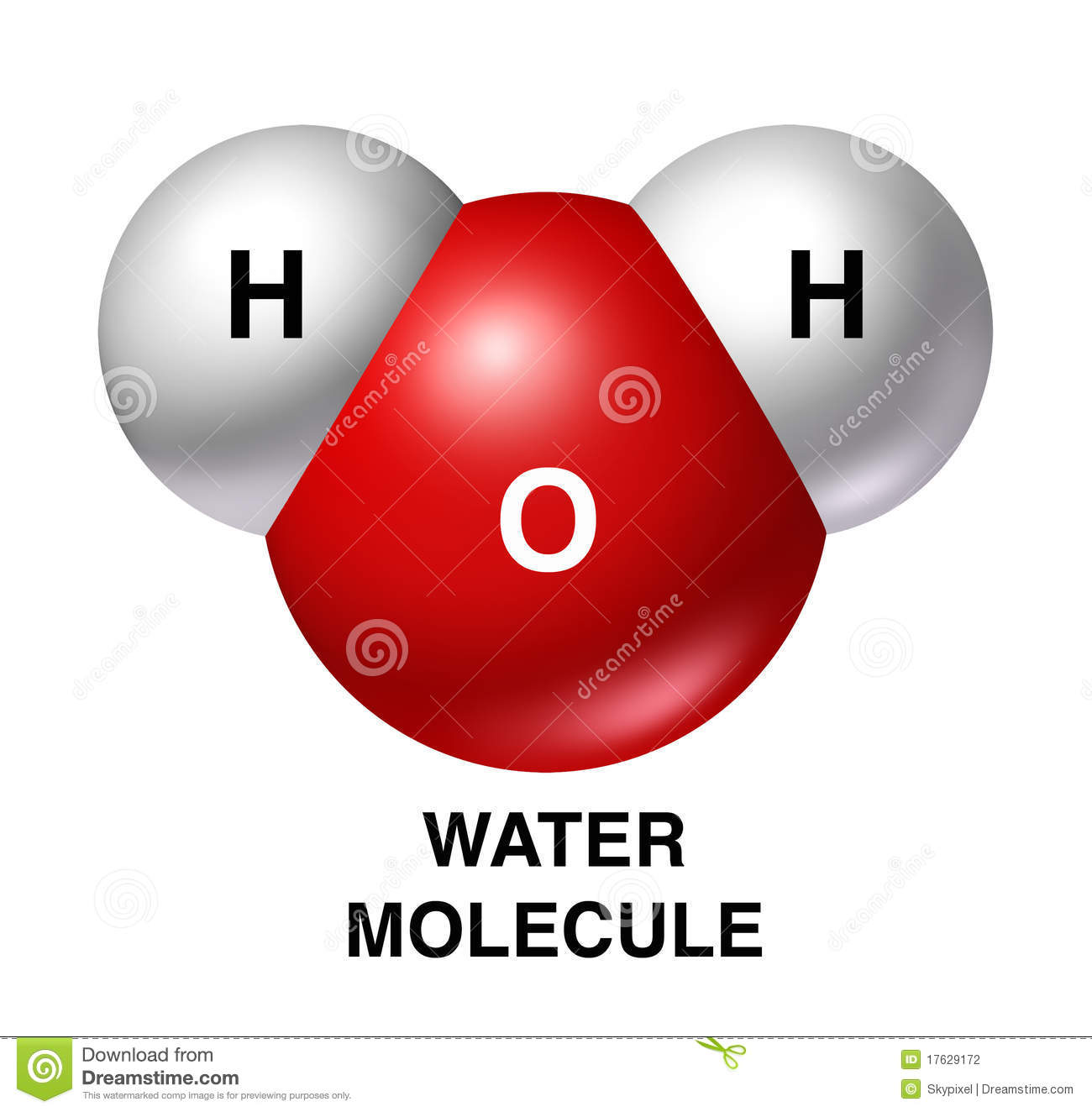
The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its hydrogen atoms.
The molecule has two poles, at which the it is colder than other regions of the molecule.
The unequal sharing of electrons gives the water molecule a slight negative charge near its hydrogen atoms and a slight positive charge near its oxygen atom.
The water molecule is neutral.
4.
Multiple Choice
As water freezes, it expands and its density decreases.
As water freezes, it takes up more hydrogen from the atmosphere, causing it to have a greater buoyancy.
As water freezes, air becomes trapped between the hydrogen bonds of water molecules.
As water freezes, it takes up more oxygen from the atmosphere, causing it to have a greater buoyancy.
5.
Multiple Choice
the oxygen end
the hydrogen end
both ends are slightly positive
neither end is positive
6.
Multiple Choice
It can be found anywhere
It freezes when it gets cold
floats when frozen
Dissolves most substances

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Adhesion, Cohesion & Surface Tension
•
6th - 8th Grade

Water Properties
•
9th Grade

Properties of Water
•
8th Grade

Ocean Sediments
•
11th - 12th Grade

Properties of Water
•
9th - 12th Grade

Properties of Water
•
9th - 12th Grade

Renewable Energy
•
11th - 12th Grade

Properties of Water
•
10th Grade