AP CHEM-Unit 8 Test
Assessment
•
Ahyoung Choi
•
Chemistry
•
11th - 12th Grade
•
5 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
27 questions
Show answers
1.
Multiple Choice
The stronger the acid, the stronger its conjugate base.
True
False
2.
Multiple Choice
CCl3COOH is a weaker acid than CH3COOH.
True
False
3.
Multiple Choice
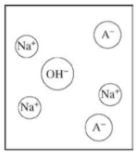
The term Ka for NH4+ refers to which equation?
NH4+(aq) + H2O(l) ⇌ NH3(aq) + H3O+(aq)
NH4+(aq) + OH-(l) ⇌ NH3(aq) + H2O(l)
NH3(aq) + H2O(l) ⇌ NH3(aq) + OH-(aq)
NH3(aq) + H3O+(aq) ⇌ NH4+(aq) + OH-(aq)
4.
Multiple Choice
Which solution has the lowest concentration of hydroxide ions?
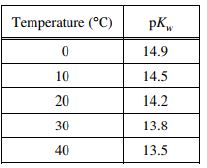
pH = 3.21
pH = 12.59
pH = 9.82
pH = 7.00
5.
Multiple Choice
What is the conjugate acid of NH3?
NH4+
NH3+
NH2-
NH3
6.
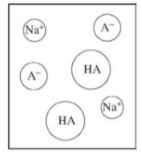
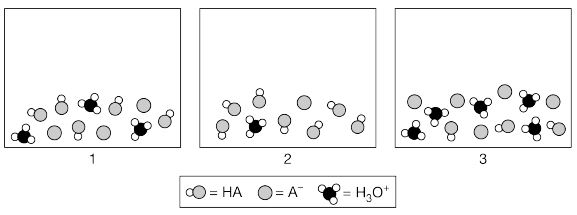
Multiple Choice
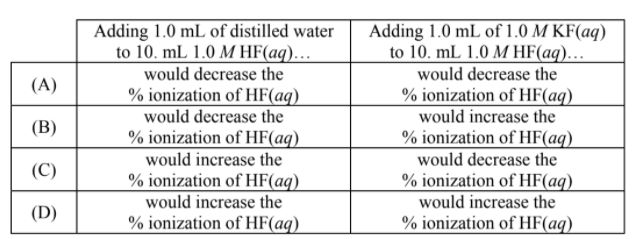
HF(aq) + H2O(l) ⇌ H3O+(aq) + F-(aq)
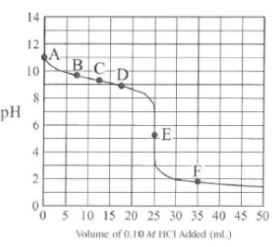
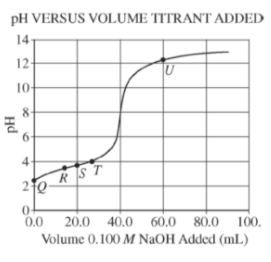
Which of the following shows what would happen to the % ionization of HF when, in two separate
experiments, 1.0 mL of distilled water and 1.0 mL of 1.0 M KF are added to a 10. mL sample of 1.0 M HF(aq)?
A
B
C
D

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Naming Acids Practice
•
10th - 12th Grade

Molarity
•
10th - 12th Grade

Acids and Bases
•
8th Grade

Acids Review
•
10th Grade

Molarity & Dilution
•
11th - 12th Grade

Naming Acids and Bases
•
9th - 12th Grade

Acids and Bases
•
9th Grade

Titration
•
10th - 12th Grade