Enthalpy
Assessment
•
Ket Llenado
•
Chemistry
•
11th Grade
•
75 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
21 questions
Show answers
1.
Multiple Choice
Define enthalpy.
The opposite of temperature
The temperature of molecule
The energy stored in the movements of the molecules
The internal energy and the product of pressure and wave
2.
Multiple Choice
What is the Law of Conservation of Energy?
Energy is equal to reactants and products
Energy can be created nor destroyed
Energy is used up but it can be replenish
Energy is conserved and preserved
3.
Multiple Choice
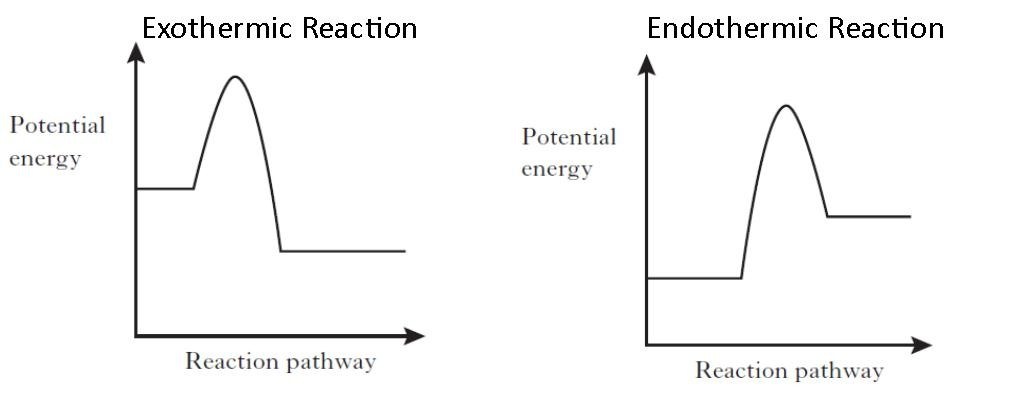
A process where the system absorbs heat from the surroundings.
Exothermic Reaction
Endothermic Reaction
Enthalpy
Entropy
4.
Multiple Choice
Study the reaction below. Is it exothermic or endothermic?
H2 + Cl2 --> 2 HCl + 1845 kJ
Exothermic Reaction
Endothermic Reaction
5.
Multiple Choice
How do you calculate the Enthalpy of Reaction?
ΔH = ΔHproducts - ΔHreactants
ΔG = ΔH -TΔS
ΔT = q / mC
E = mc2
6.
Multiple Choice
A solute dissolves in water and gets cold.
Exothermic with positive ΔH
Exothermic with negative ΔH
Endothermic with positive ΔH
Endothermic with negative ΔH

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Balancing Equations
•
10th Grade

Balancing Equations
•
8th Grade

Types of Chemical Reaction
•
10th Grade

Classifying Reactions
•
10th - 11th Grade

Fertilizers
•
1st - 5th Grade

Chemical Reactions
•
8th Grade

Balancing Chemical Equations
•
KG

Reversible and Irreversible Reaction & Chemical Equilibrium
•
9th Grade