
Titration 101
Assessment
•
Jordan White
•
Chemistry
•
9th - 12th Grade
•
3 plays
•
Hard
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
5 questions
Show answers
1.
Multiple Choice
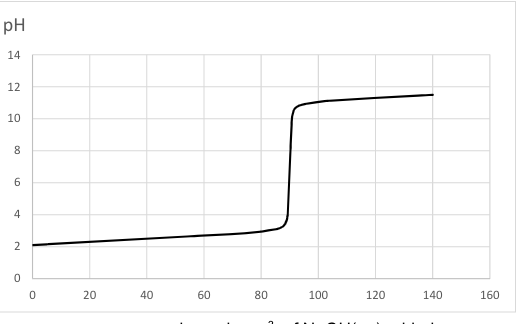
Look at the image above. This is the reaction of:
A strong base being added to a strong acid
A weak base being added to a strong acid
A strong base being added to a weak acid
A strong acid being added to a weak base
2.
Multiple Choice
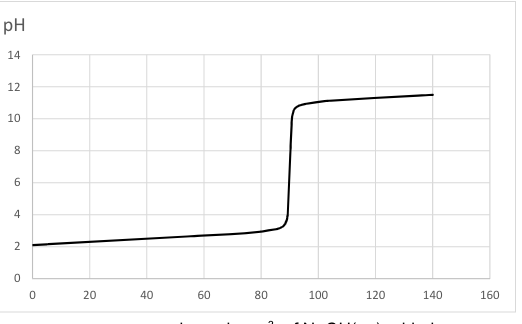
Assuming the initial reagent in the flask is a strong monoprotic acid, estimate its concentration.
0.1 mol/dm3
0.01 mol/dm3
0.2 mol/dm3
0.02 mol/dm3
3.
Multiple Choice
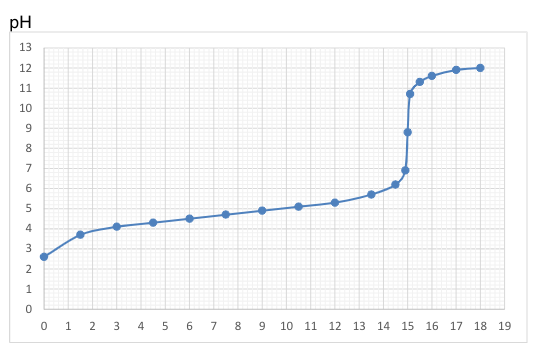
Which of the following statements about this titration is accurate?
Strong acid was added to weak base
Strong base was added to weak acid
Weak acid was added to strong base.
Strong base was added to strong acid.
4.
Multiple Choice
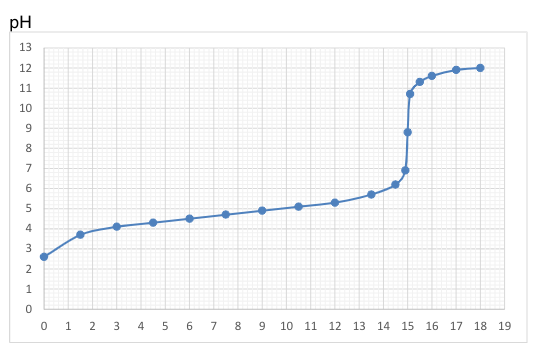
Calculate the initial concentration of 25ml of the reagent H-X where the substance added had a concentration of 0.1 mol/dm3.
0.06 mol/dm3
0.6 mol/dm3
0.16 mol/dm3
0.02 mol/dm3
5.
Multiple Choice
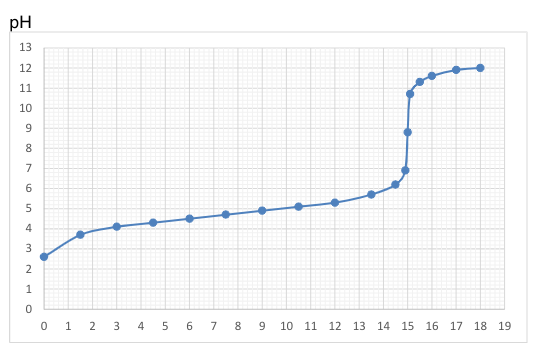
Determine the pKa of the reactant.
8.8
15.0
7.5
4.8

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Acids and Alkalis
•
7th Grade

Acids and Bases
•
9th - 12th Grade

Acids Review
•
10th Grade

Molarity & Dilution
•
11th - 12th Grade

Acids, Bases and Salts
•
10th Grade

Acids & Bases
•
9th - 12th Grade

Acids and Bases
•
9th Grade

Titration
•
10th - 12th Grade