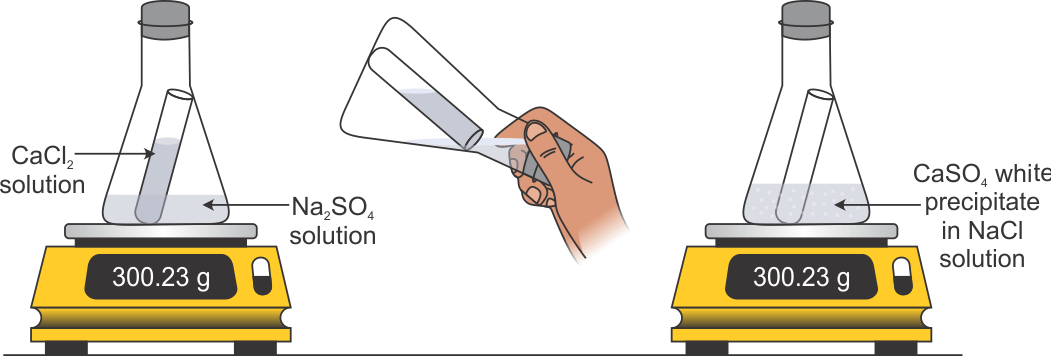
Modeling Conservation of Mass
Assessment
•
undefined undefined
•
Science
•
6th - 8th Grade
•
75 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
15 questions
Show answers
1.
Open Ended
Does this chemical equation follow the Law of Conservation of Mass? Use evidence from the equation to support your response.
H2 + O2 → H2O
Evaluate responses using AI:
OFF
Answer explanation
This equation does NOT follow the Law of Conservation of Mass.
It does not follow the law of conservation of mass because it is not balanced. There are 2 hydrogen atoms on the reactant side and 2 hydrogen atoms on the product side. There are 2 oxygen atoms on the reactant side but only 1 oxygen atom on the product side.
Since there is not the same number of oxygen atoms on both sides, the equation is not balanced. The law of conservation of mass states that mass cannot be created or destroyed. In order to follow this law, the equation needs to have the same number of each element on both sides of the equation.
2.
Multiple Choice
How many total atoms of chlorine are there in 2NaCl2?
1
2
3
4
Answer explanation
The coefficient in front Na applies to both Na and Cl. (2 x 2 = 4)
3.
Multiple Choice
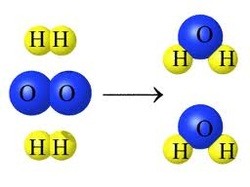
Which equation supports this model?
2H2 + O2 → 2H2O
H2 + O2 → 2H2O
2H2O2 → 2H2O
2H2 + O2 → H2O
Answer explanation
Look at the model. There are 2 molecules of H2 and 1 molecules of O2 on the reactant side. On the product side, you have 2 molecules of H2O.
4.
Multiple Choice
Balance this equation:
Al + O2 → Al2O3
2Al + O2 → Al2O3
4Al + O2 → 2Al2O3
4Al + 3O2 → 2Al2O3
2Al + 2O2 → 4Al2O3
5.
Open Ended
Balance this equation and then calculate the total atomic mass of the reactants and products.
H2 + O2 → H2O
Evaluate responses using AI:
OFF
Answer explanation
Balanced equation: 2H2 + O2 → 2H2O
Reactants:
H = 4 atoms x 1 = 4
O = 2 atoms x 16 = 32
Total mass: 36 amu
Products:
H = 4 atoms x 1 = 4
O = 2 x 16 = 32
Total mass: 36 amu
6.
Multiple Choice
Which number represent a coefficient in this equation?
Cl6 + Na6 → 3Cl2Na2
2
3
6
8
Answer explanation
Coefficient is the number in front of the substance. It tells you how many molecules (or groups) of the substance you have.

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Chemical Equations
•
6th - 8th Grade

Law of Conservation of Mass
•
7th Grade

Law of Conservation of Mass
•
7th Grade

Chemical Equations
•
8th Grade

Types of Chemical Reactions
•
8th - 10th Grade

Chemical Reactions
•
8th - 10th Grade

Evidence of Chemical Reactions
•
6th - 9th Grade

Chemical Reactions
•
8th Grade