Collision Theory
Assessment
•
Anete Biteniece
•
Chemistry
•
10th - 12th Grade
•
1 plays
•
Hard
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
16 questions
Show answers
1.
Multiple Choice
What is collision theory?
Molecules must collide in the correct orientation with enough energy to bond.
Molecules need enough energy to collide and react.
Atoms constantly collide and react.
The minimum energy needed for atoms to react
2.
Multiple Choice
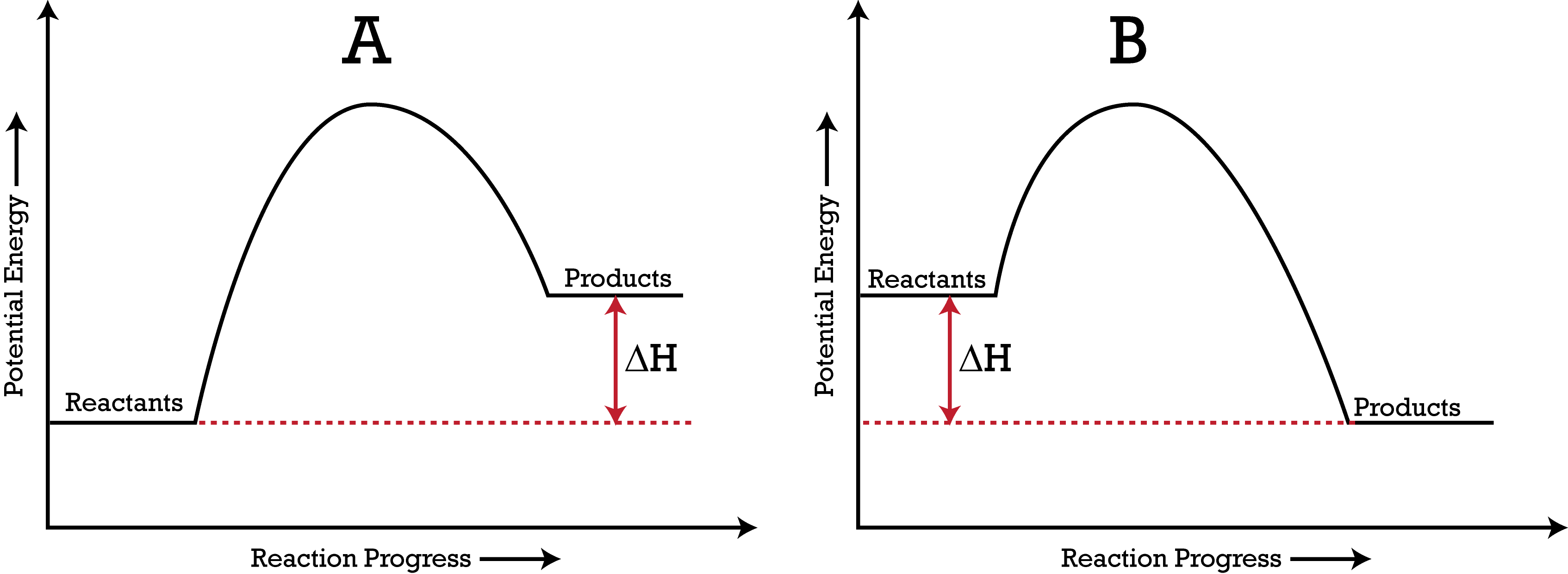
Which PE Diagram represents an endothermic reaction?
A
B
3.
Multiple Choice
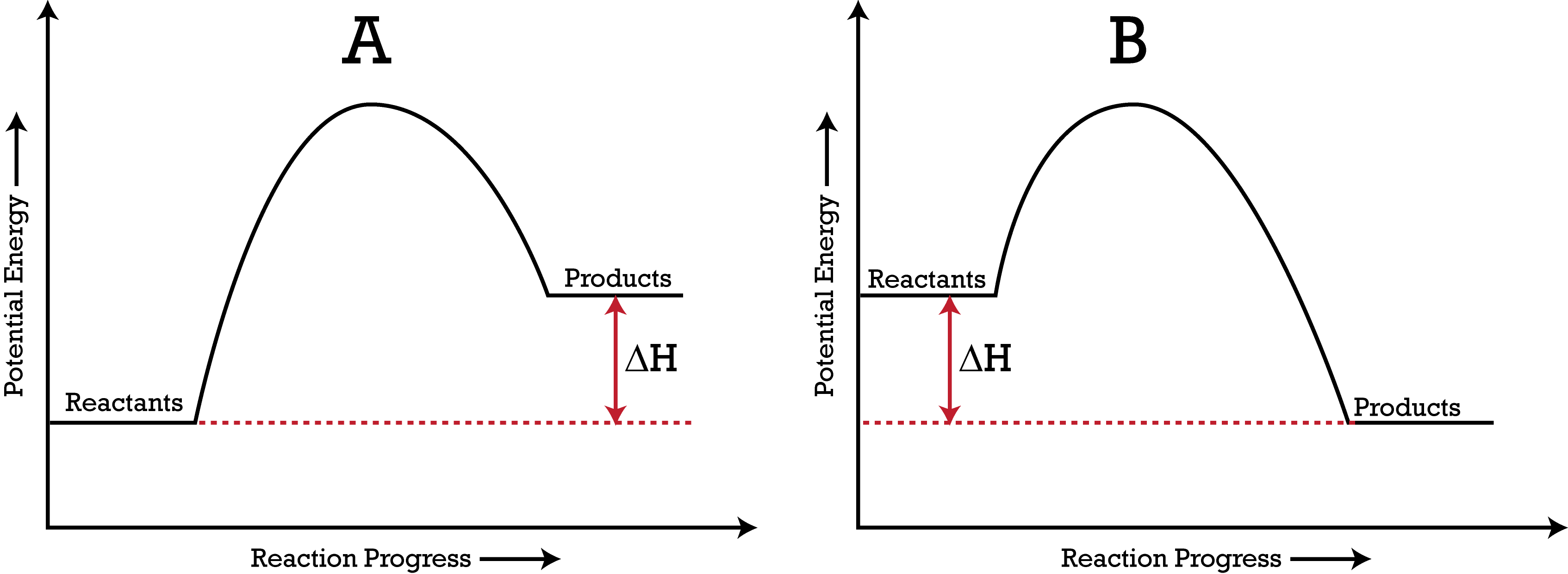
Which PE Diagram represents an exothermic reaction?
A
B
4.
Multiple Choice
What is the rate of reaction?
How much energy is needed for a reaction to occur.
The energy required to break a bond.
The time it takes for a reaction to occur.
Collision Theory
5.
Multiple Choice
What is the purpose of a catalyst?
Helps to slow down a reaction
Raises the activation energy
Lowers the activation energy
Is consumed by the reaction
6.
Multiple Choice
What factors effect rate of reaction?
Temperature, Concentration, Pressure and Energy
Surface Area, Concentration, Energy and Pressure
Temperature, Pressure, Concentration and Surface area
Pressure, Surface area, Density and Energy

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Balancing Equations
•
10th Grade

Equilibrium
•
11th - 12th Grade

Reaction Kinetics
•
University - Professi...

Balancing Equations
•
10th Grade

Endothermic and Exothermic Reactions
•
9th Grade

Classifying Reactions
•
10th - 11th Grade

Aldehydes and Ketones
•
University

Chemical Reactions
•
8th Grade