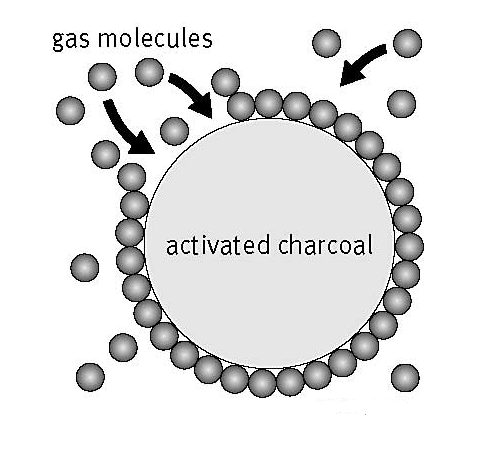
Surface Chemistry
Assessment
•
Vinod Garg
•
Science, Chemistry
•
12th Grade
•
85 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
15 questions
Show answers
1.
Fill in the Blank
What kind of process is adsorption?
2.
Multiple Select
Entropy during adsorption ...........
Decreases
Increases
Remain Same
Cannot say
3.
Multiple Choice
Peptisation is a process of
Precipitation of colloid
Dispersion of precipitate
purification of colloids
Movement of colloid under electric potential
4.
Fill in the Blank
The difference in potential of fixed and mobile layer is called ..........
5.
Multiple Choice
Extent of physisorption increases with
decrease in surface area
increase in temperature
decrease in temperature
decrease in strength of van der waal forces
6.
Multiple Choice
In physisorption adsorbent does not show specificity for any particular gas because ______________.
involved van der Waals forces are universal
it is a reversible process
Enthalpy is low
Gases behave as ideal gases

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Day and Night
•
KG

Sorting Materials Into Groups
•
5th - 6th Grade

States of Matter
•
6th Grade

Nature of Matter
•
6th Grade

Matter
•
4th Grade

Matter in our Surroundings
•
9th Grade

States Of Matter
•
3rd - 4th Grade

Matter
•
3rd - 5th Grade