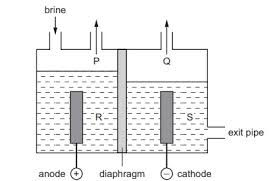
Electrolysis of Brine
Assessment
•
Kim Bishop
•
Science
•
5th Grade
•
43 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
10 questions
Show answers
1.
Multiple Choice
Which one of the following metal is not extracted by electrolysis?
Aluminium
Iron
Sodium
Potassium
2.
Multiple Choice
cathode
anode
3.
Multiple Choice
Hydrogen
Oxygen
Chlorine
Sodium
4.
Multiple Choice
In the electrolysis of brine what product do we get at the cathode?
hydrogen gas
oxygen gas
sodium metal
chlorine gas
5.
Multiple Choice
Which are the useful products of electrolysis of brine?
sodium hydroxide, chlorine and hydrogen
sodium metal, oxygen and chlorine
sodium metal, chlorine and sodium hydroxide
sodium hydroxide, oxygen and hydrogen
6.
Multiple Choice
So the ions can move
So the electrons can move
So that it doesn't get too hot
So the fish are ok

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Electrolysis of Brine
•
5th Grade

Ionic Bonding
•
10th Grade

Metal Reactions
•
8th - 10th Grade

Reactivity of Metals
•
9th Grade

Types of Reactions
•
8th Grade

Thermal Decomposition
•
6th - 7th Grade

Molarity and Molality
•
9th - 12th Grade

Periodic Trends
•
10th - 12th Grade