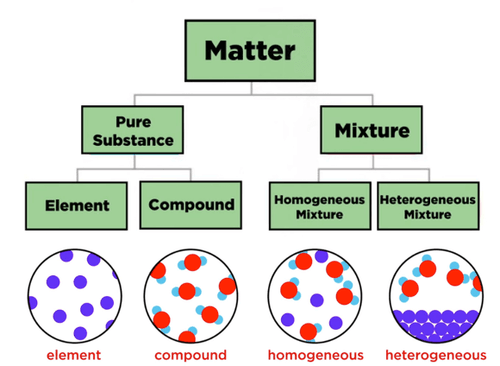
Pure Substances Vs. Mixtures
Assessment
•
Claryliz Peralta
•
Chemistry
•
10th - 11th Grade
•
719 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
15 questions
Show answers
1.
Multiple Select
[check all that apply] Pure substances include:
Elements
Homogeneous Mixtures
Compounds
Heterogeneous Mixtures
2.
Multiple Choice
An element is?
Chemically combined
Pure substances that can be broken down
Pure substances that cannot be broken down into simpler substances
One or more elements chemically combined.
3.
Multiple Select
[check all that apply] An compound is?
Chemically combined
Pure substances that can be broken down
Pure substances that cannot be broken down into simpler substances
One or more elements chemically combined.
4.
Multiple Choice
The properties of a compound are different from the elements that make them up.
True
False
5.
Multiple Choice

Oxygen is a
Element
Compound
6.
Multiple Choice
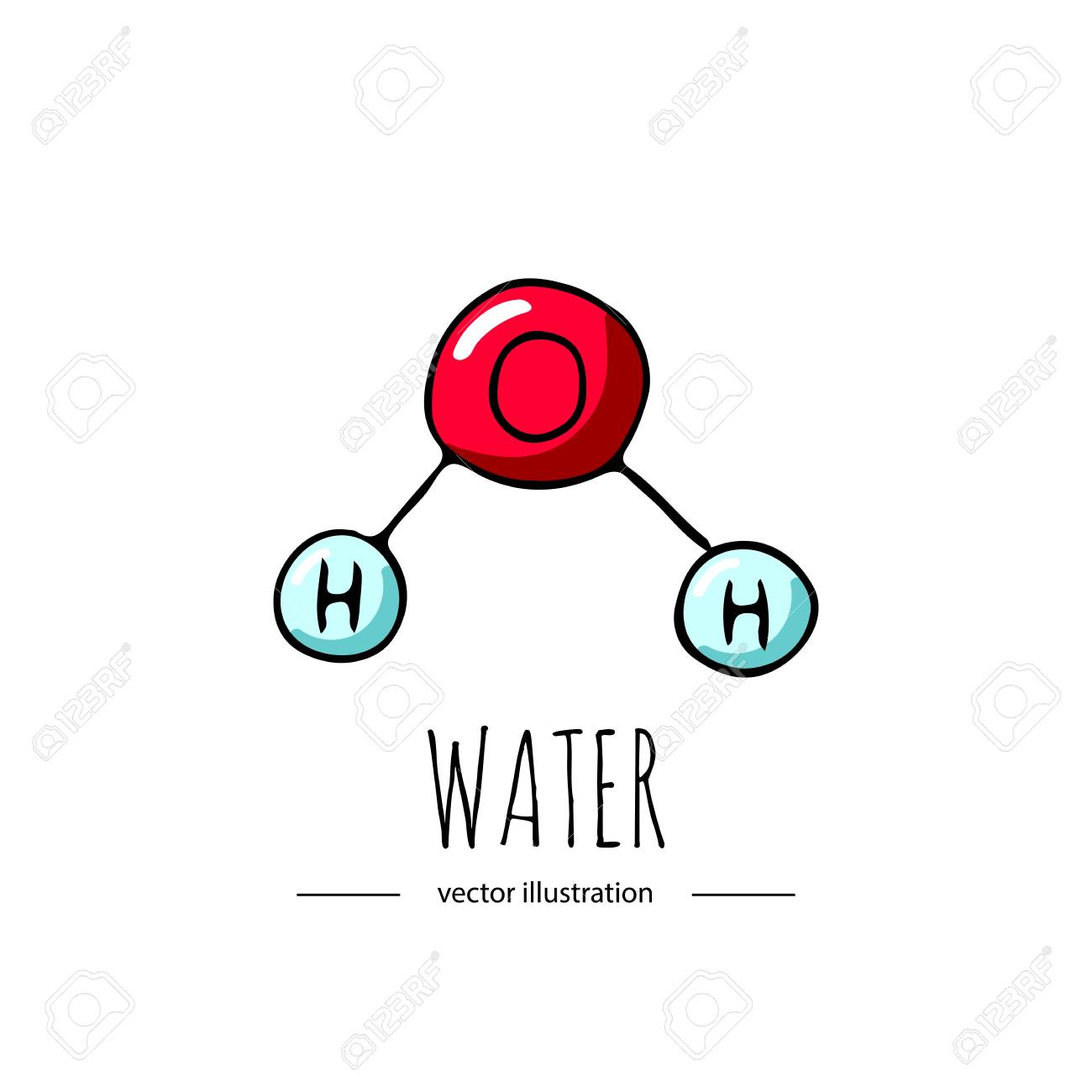
Water (H2O) is a
Element
Compound

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Elements & Compounds
•
6th Grade

Elements, Compounds and Mixtures
•
8th Grade

Counting Atoms
•
8th Grade

Classifying Matter
•
9th - 10th Grade

Elements, Compounds, Mixtures
•
9th - 12th Grade

Counting Atoms
•
9th - 12th Grade

Elemental and Compound Molecules
•
7th Grade

Characteristics of Matter
•
10th Grade




