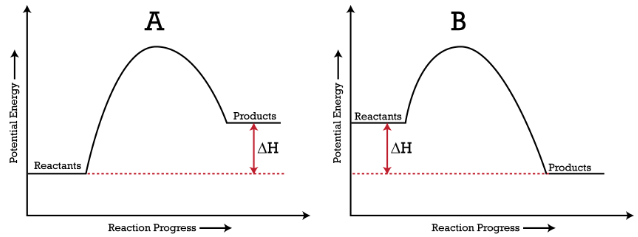
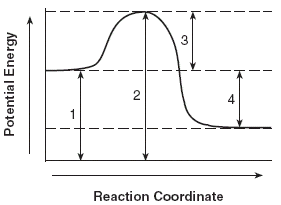
Endothermic & Exothermic
Assessment
•
Derek Brumleve
•
Chemistry
•
9th - 11th Grade
•
3 plays
•
Easy
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
10 questions
Show answers
1.
Multiple Choice

exothermic
endothermic
2.
Multiple Choice

taken in or absorbed
given out or released
3.
Multiple Choice

It is an exothermic reaction
It is an endothermic reaction
It is made of ice
It is dissolving your mouth
4.
Multiple Choice
endothermic
exothermic
5.
Multiple Choice
endothermic reaction
exothermic reaction
6.
Multiple Choice

_____ reactions usually feel hot!
endothermic
exothermic

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Chemical Equations
•
8th Grade

Types of Reactions
•
10th - 12th Grade

Balancing Chemical Equations
•
8th - 12th Grade

Balancing Equations
•
10th Grade

Classifying Reactions
•
10th - 11th Grade

Types of Chemical Reactions
•
9th - 12th Grade

Chemical Reactions
•
8th Grade

Chemical Equations and Balancing
•
10th - 12th Grade