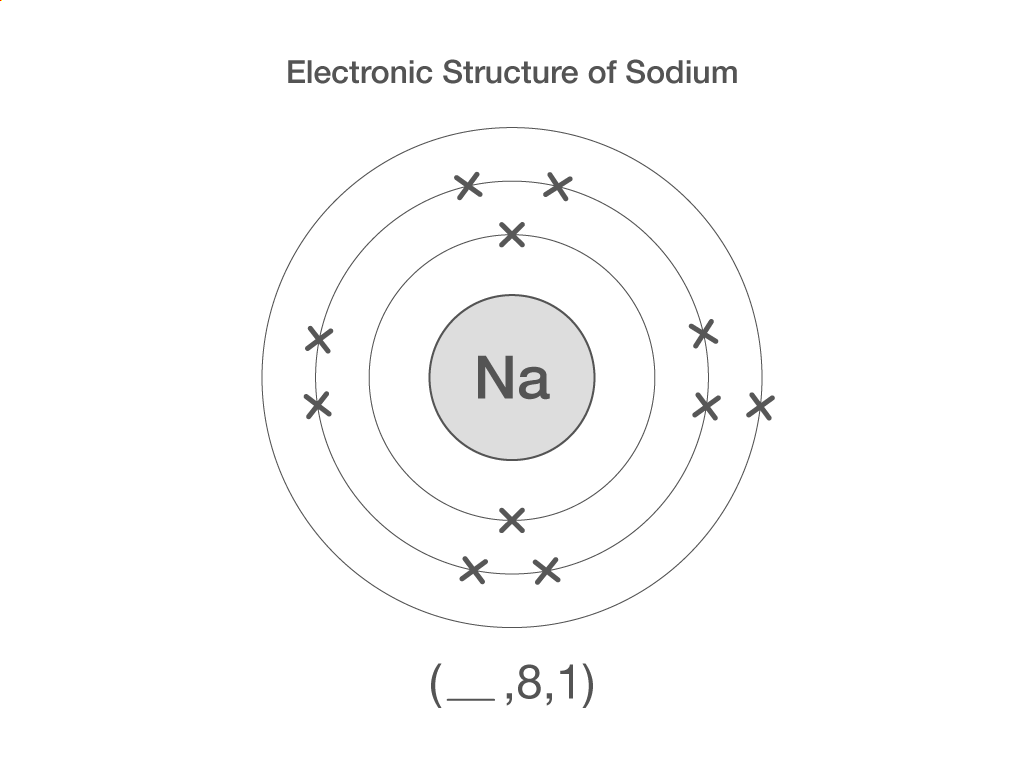
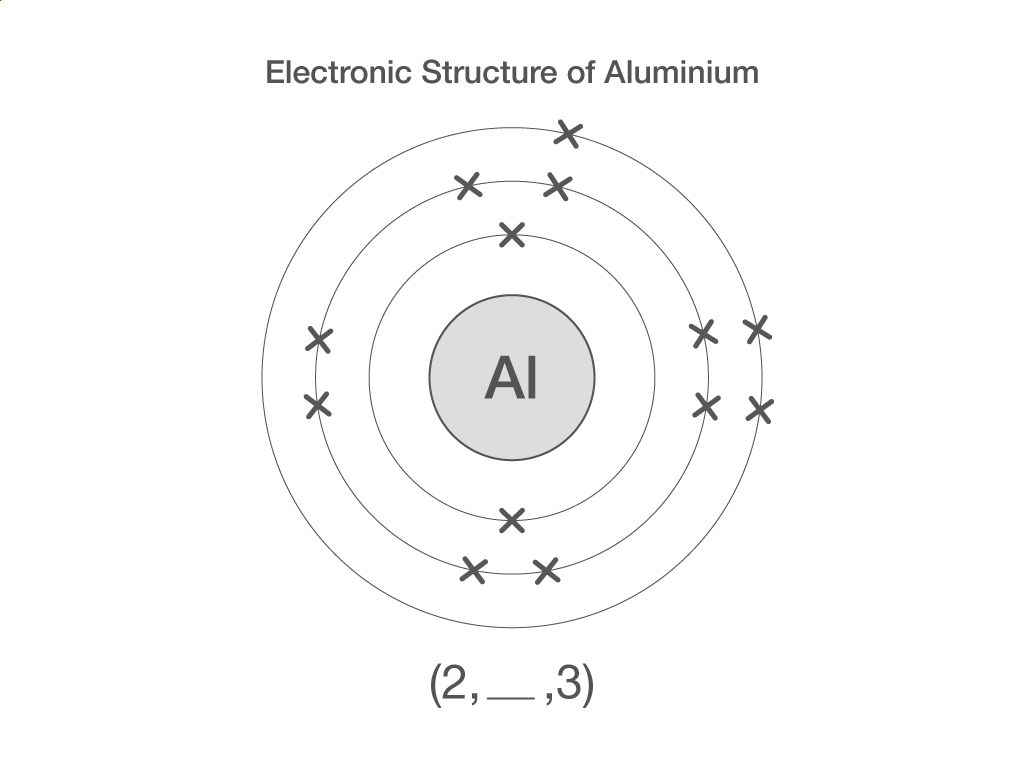
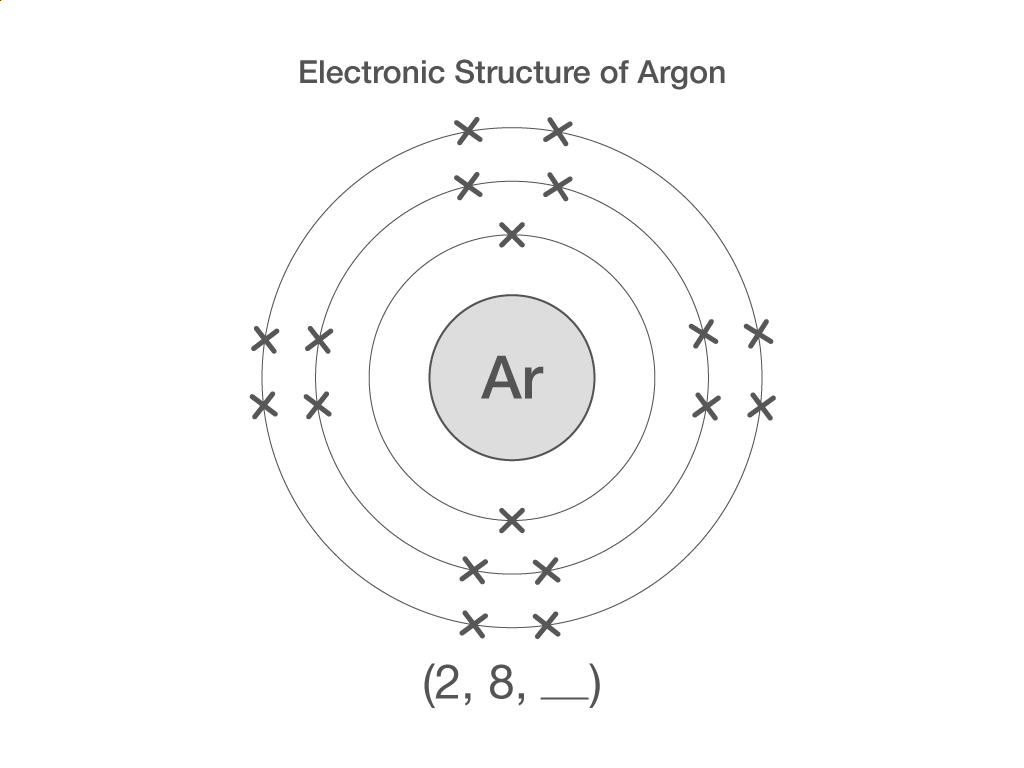
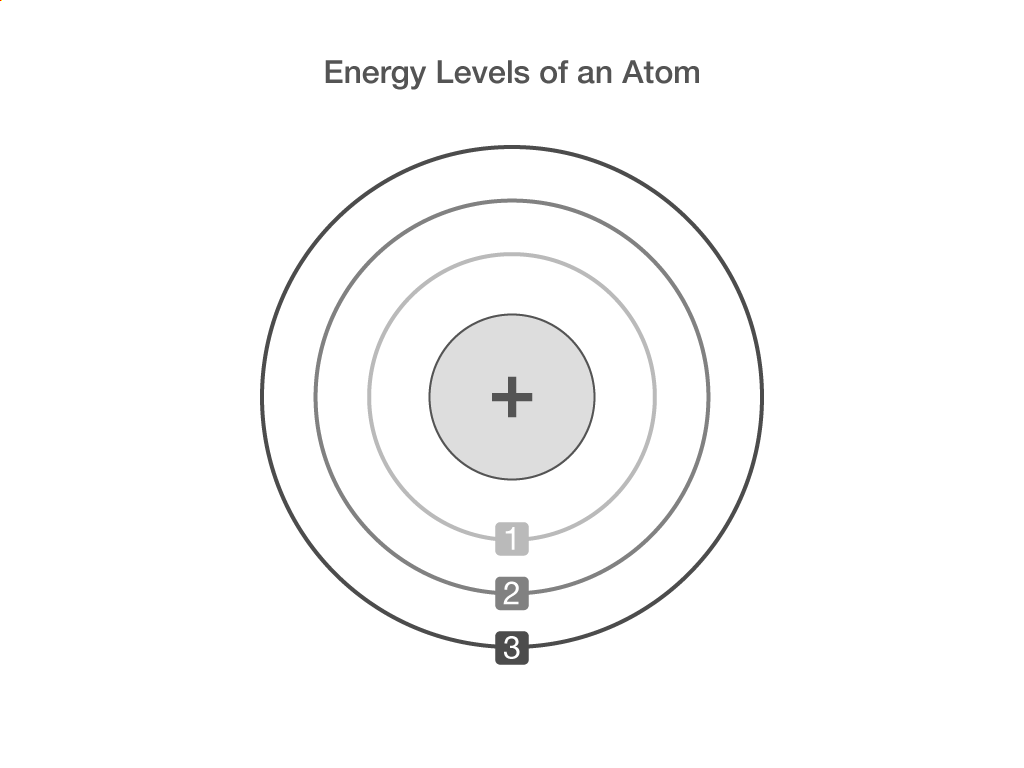
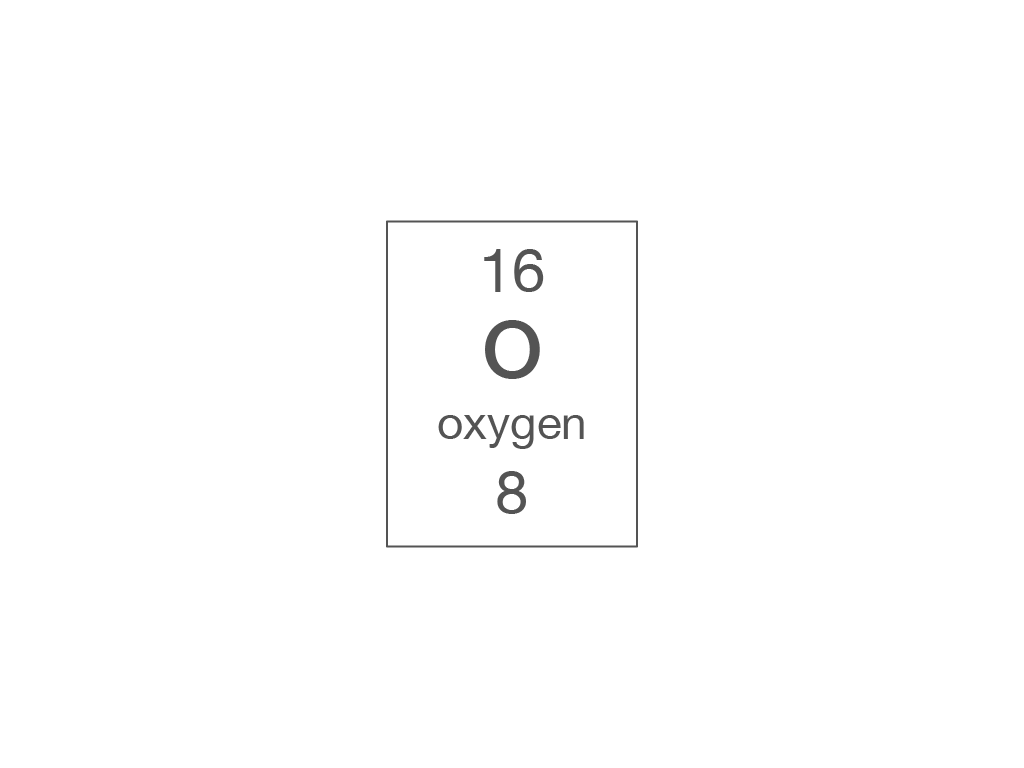
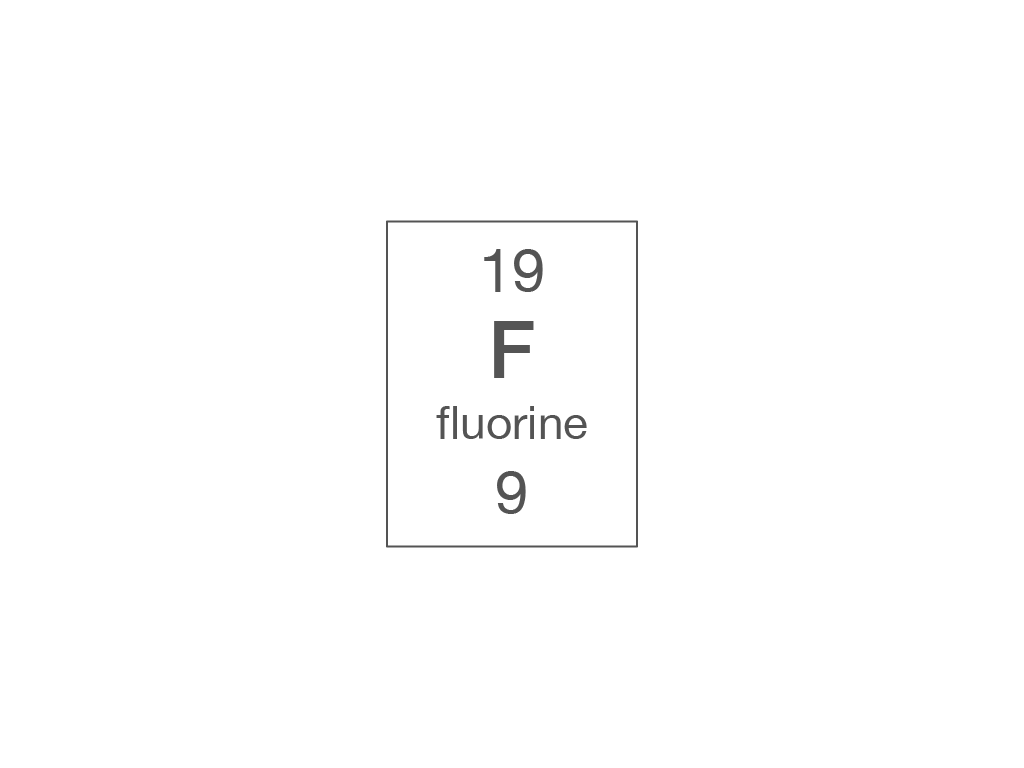
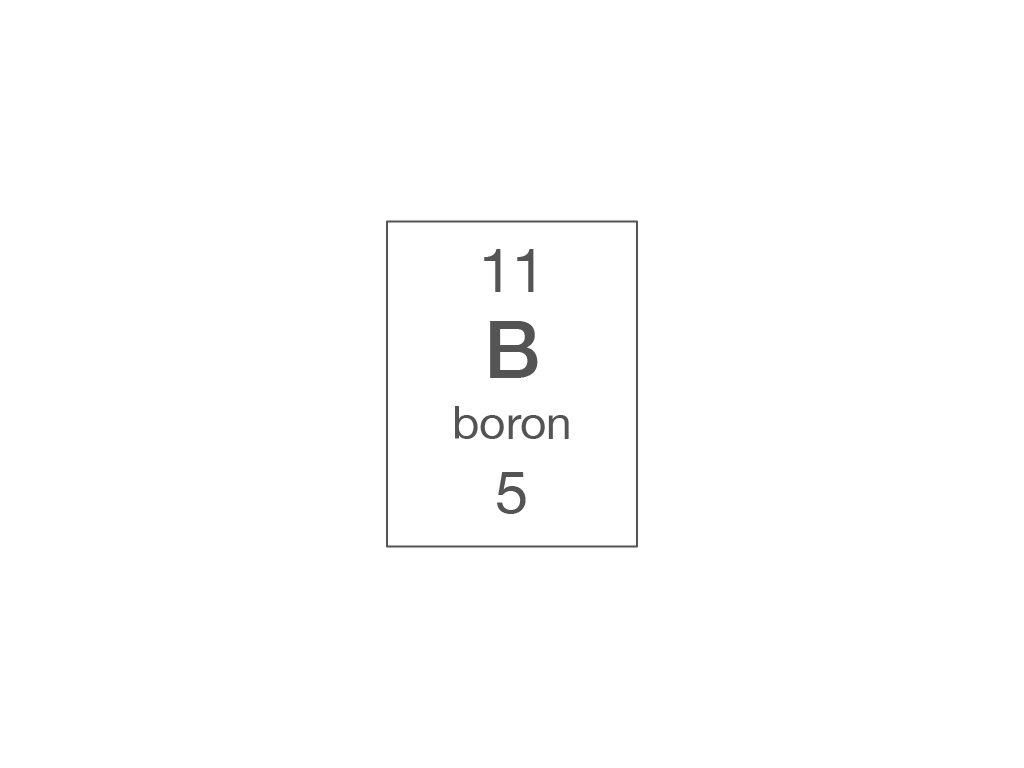
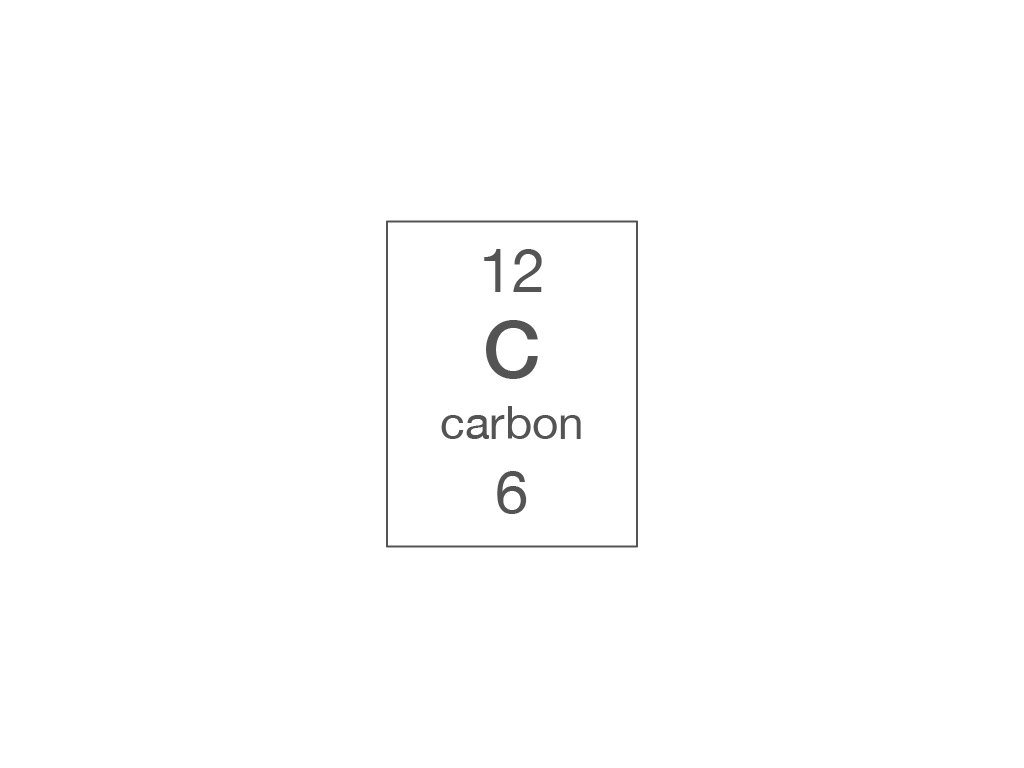
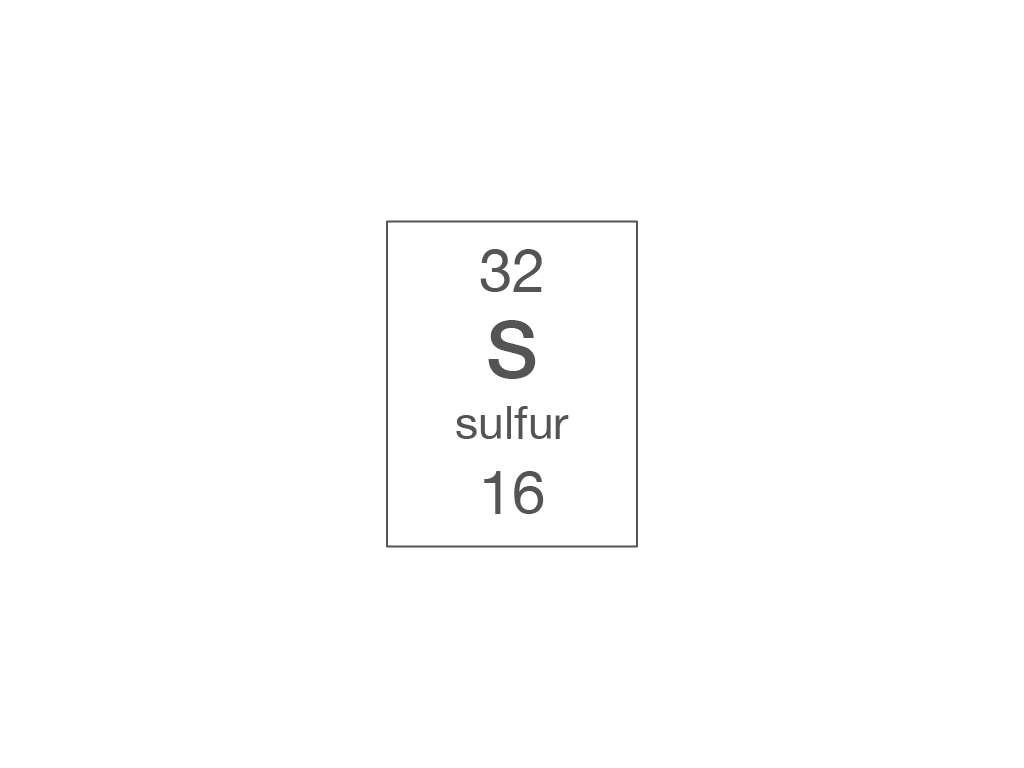
Electronic structure
Assessment
•
Michael Spowart
•
Chemistry, Science
•
9th - 10th Grade
•
314 plays
•
Easy
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
25 questions
Show answers
1.
Multiple Choice
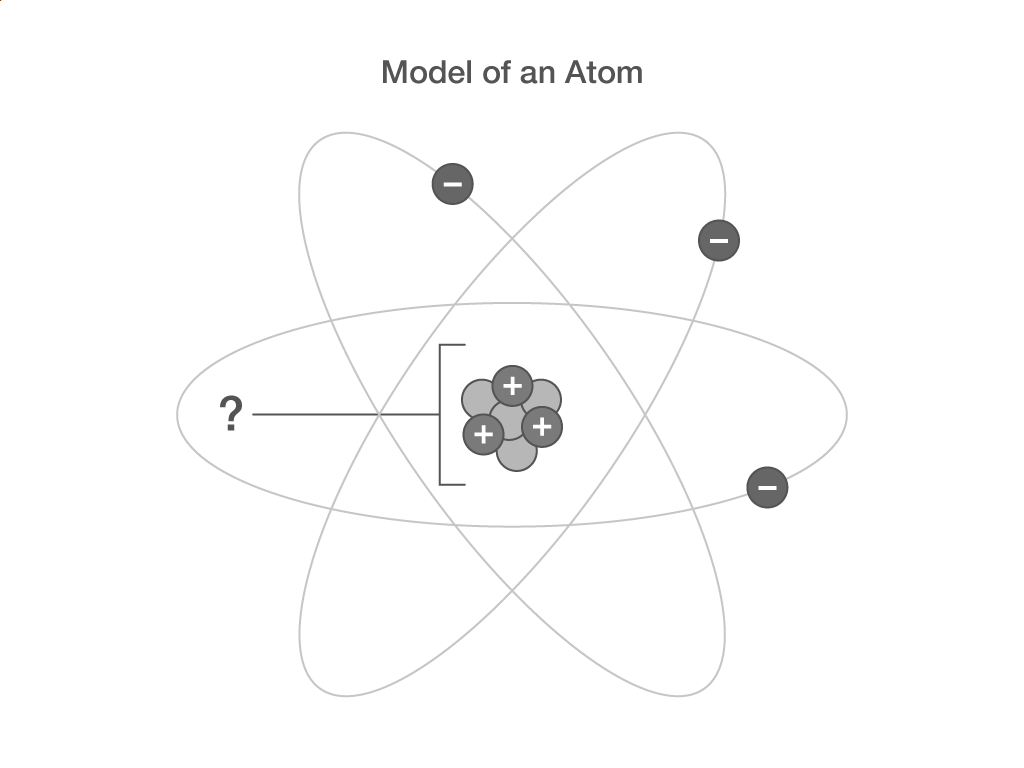
What is the centre of an atom called?
nucleus
core
atomic centre
2.
Multiple Select
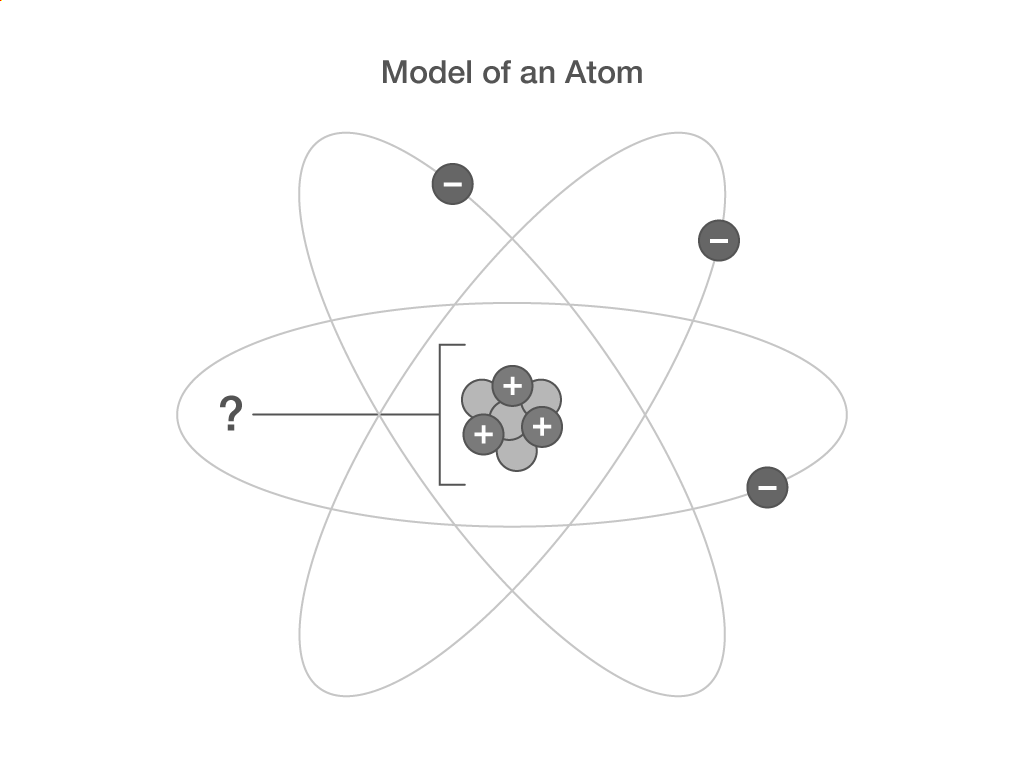
Which two sub-atomic particles are in the nucleus of an atom?
protons
electrons
neutrons
3.
Multiple Choice
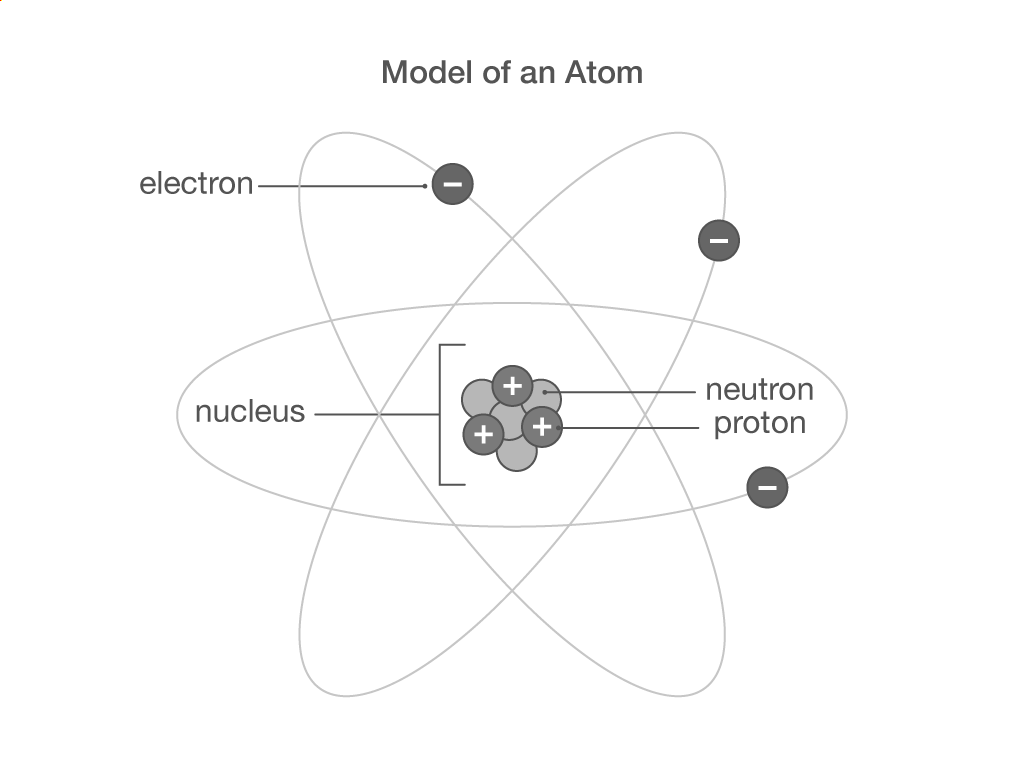
Why does an atom have no overall charge?
An atom has an equal number of positive electrons and negative protons.
An atom has an equal number of positive protons and negative electrons.
There are no charged sub-atomic particles in an atom to give it an overall charge.
4.
Multiple Choice
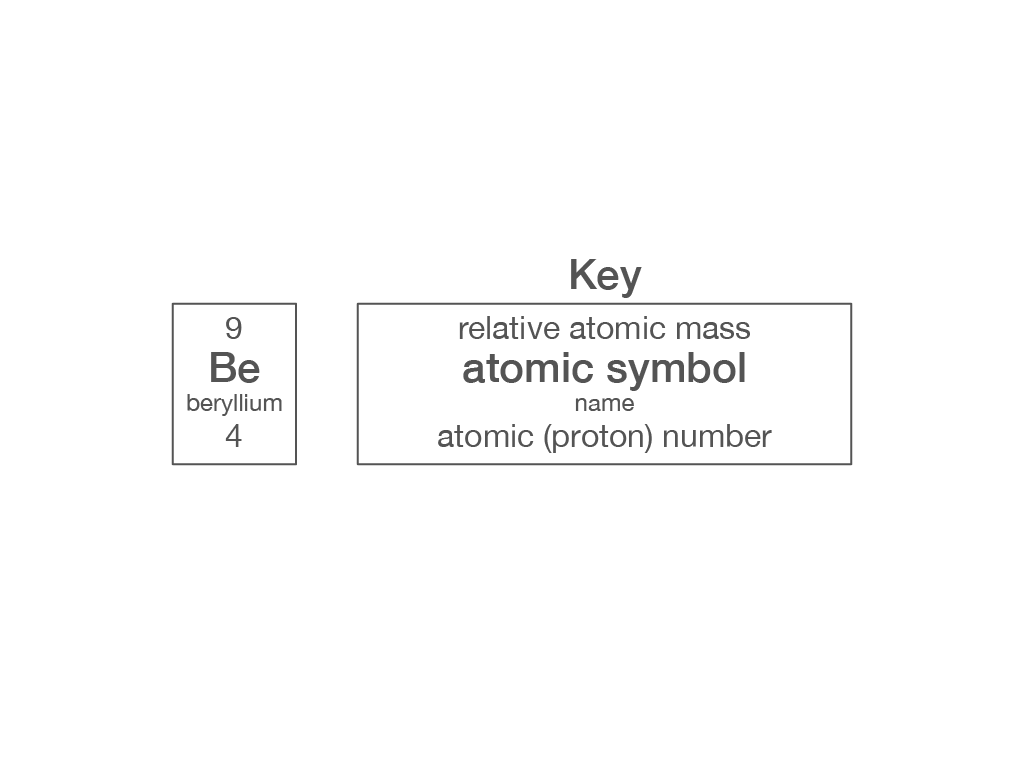
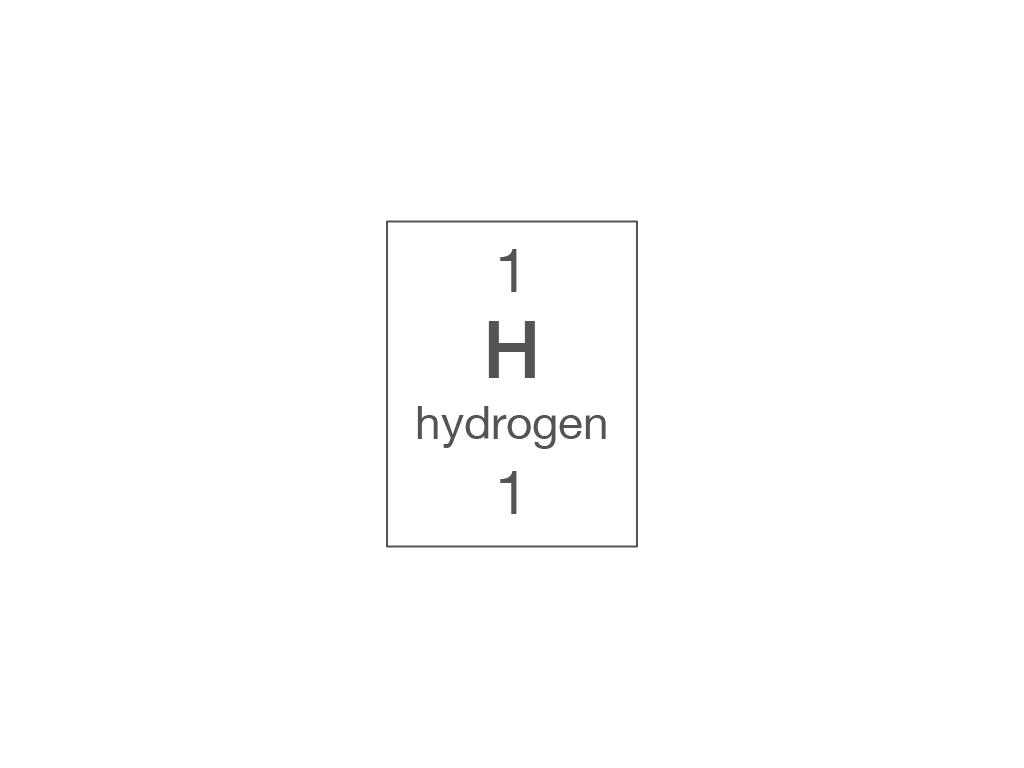
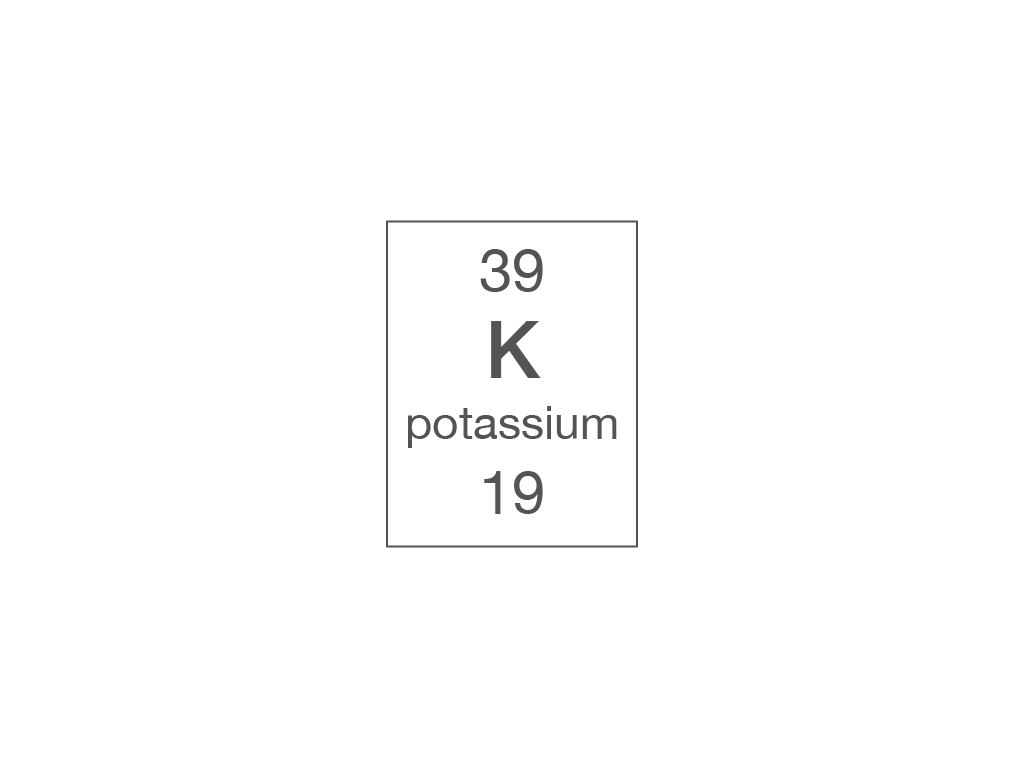
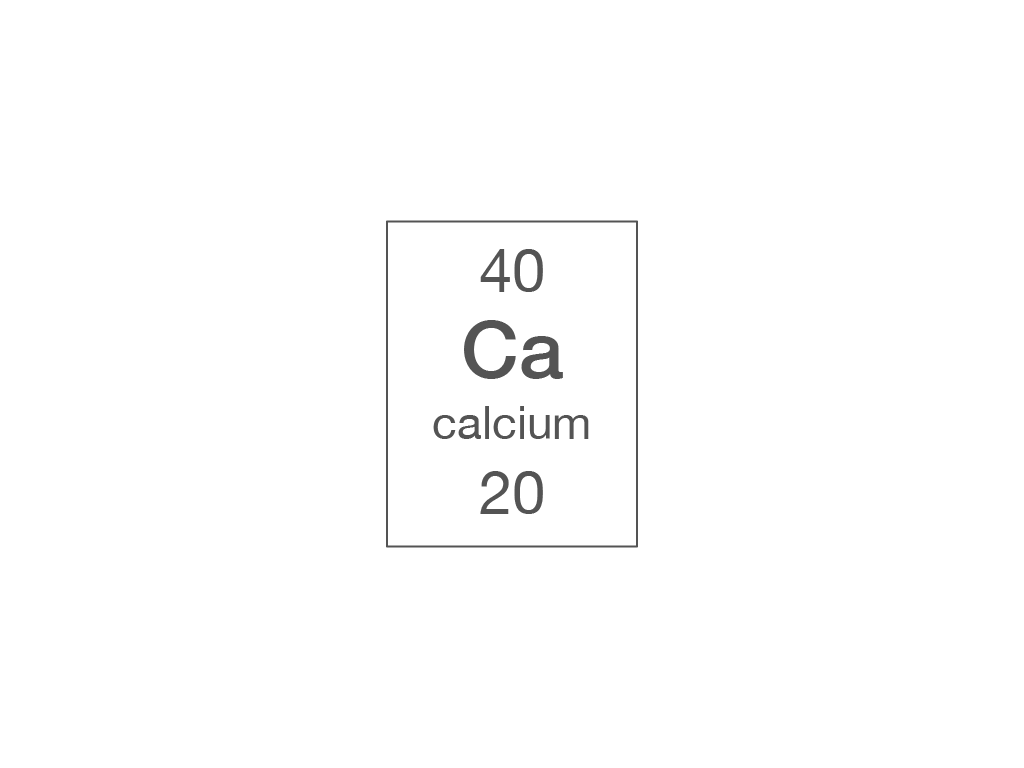
Which piece of information from the periodic table can you use to work out the number of electrons in an atom?
relative atomic mass
atomic symbol
element name
atomic (proton) number
5.
Multiple Choice
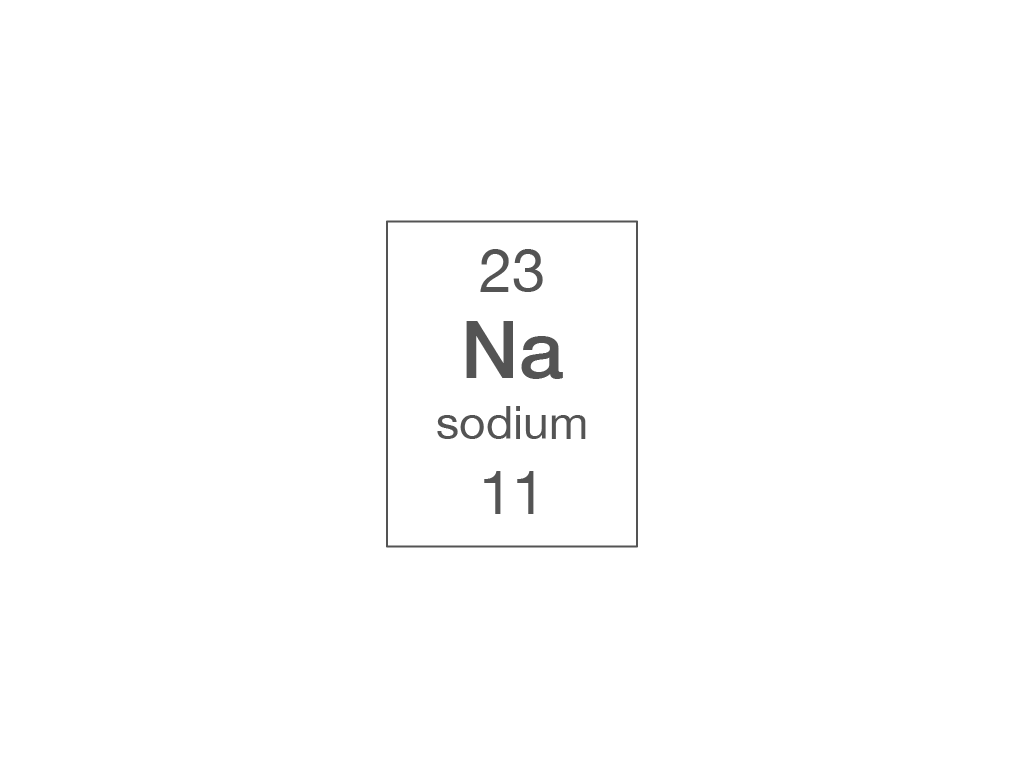
How many electrons are there in a sodium atom?
11
23
12
34
6.
Multiple Choice
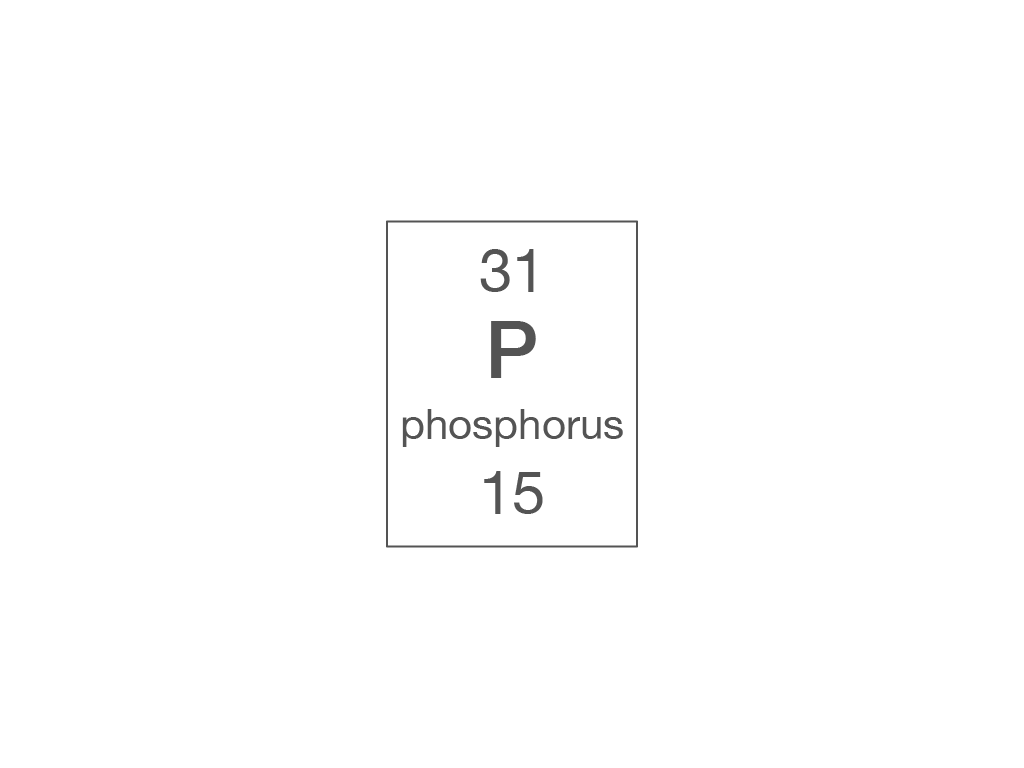
How many electrons are there in a phosphorus atom?
15
31
16
47

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Atomic Structure Pop Quiz
•
8th Grade

Electron Configuration
•
8th - 10th Grade

Nuclide Notation
•
9th - 12th Grade

Atoms and the Periodic Table
•
7th - 11th Grade

Models of the Atom
•
8th - 10th Grade

Alcohols
•
1st Grade

Atomic Structure
•
9th - 12th Grade

Atomic Number, Mass Number and Isotopes
•
9th - 12th Grade