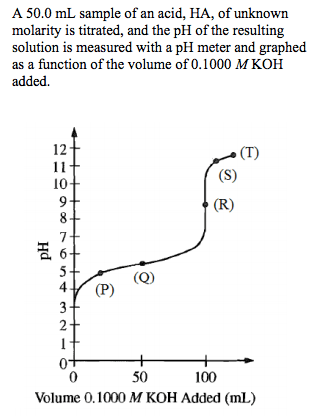
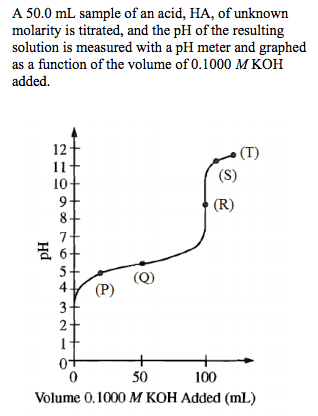
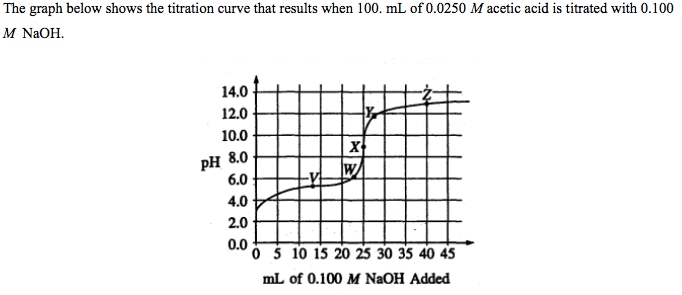
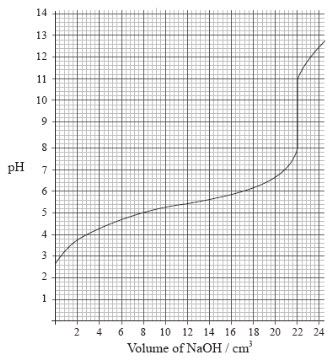
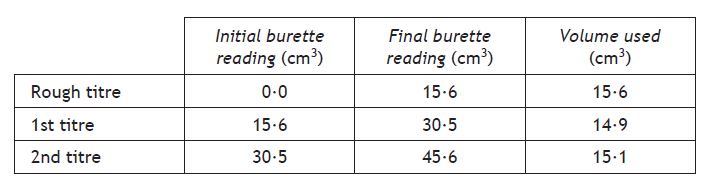
Titrations
Assessment
•
Christina Davis
•
Chemistry
•
10th Grade
•
38 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
30 questions
Show answers
1.
Multiple Choice
What allows us to know when the end point has been reached?
Bubbles
Feels warm
indicator changing color
Turns to a solid
2.
Multiple Choice
salt + hydrogen
salt + water
salt + carbon dioxide + water
salt
3.
Multiple Choice
Amount of Oxygen Ions
Amount of Hydrogen Ions
The amount of salt in a solution
The density
4.
Multiple Choice

What is this piece of apparatus called
Pipette
Burette
Janette
Cuvette
5.
Multiple Choice
Acid
Base
Neutral
Acid and Base
6.
Multiple Choice
Why do we perform a titration?
To determine the concentration of an unknown solution
To see if a reaction will occur between an acid and base
To find the mass of an unknown acid
To find the molar mass of an unknown solution
To calculate the viscosity of the solution

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Naming Acids Practice
•
10th - 12th Grade

Molarity
•
10th - 12th Grade

Acids and Bases
•
8th Grade

Acids Review
•
10th Grade

Molarity & Dilution
•
11th - 12th Grade

Naming Acids and Bases
•
9th - 12th Grade

Acids and Bases
•
9th Grade

Titration
•
10th - 12th Grade