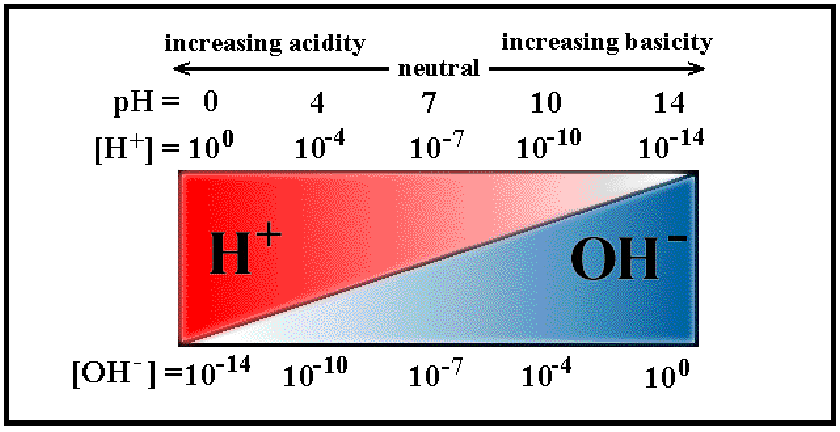
Strong Acids and Bases
Assessment
•
Katherine Dirksen
•
Chemistry
•
9th - 12th Grade
•
114 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
21 questions
Show answers
1.
Multiple Choice
HCl, HBr, HI
hydrochloric acid, hydrobromic acid, hydroiodic acid
chloric acid, bromic acid, iodic acid
chlorous acid, bromous acid, iodous acid
hydrochlorous acid, hydrobromous acid, hydroiodous acid
2.
Multiple Choice
--sulfuric acid--
--nitric acid--
H2SO, HNO
H2SO3, HNO3
H2S, H3N
H2SO4, HNO3
3.
Multiple Choice
HClO4, HClO3
chlorous acid, hypochlorous acid
perchloric acid, chloric acid
chloric acid, perchloric acid
hypochlorous acid, chlorous acid
4.
Multiple Choice
do not break apart into ions (non-electrolyte)
partially break apart into ions (weak electrolyte)
completely break apart into ions (non-electrolyte)
completely break apart into ions (strong electrolyte)
5.
Multiple Choice
all acids and bases are weak
if not classified as strong, the acid/base is considered weak
all acids and bases are strong
there are more strong acids and bases than there are weak acids/bases
6.
Multiple Choice
--hydrochloric acid--
--hydrobromic acid--
--hydroiodic acid--
HClO3, HBrO3, HIO3
HCl, HBr, HI
HClO4, HBrO4, HIO4
HClO, HBrO, HIO

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Naming Acids Practice
•
10th - 12th Grade

Molarity
•
10th - 12th Grade

Acids and Bases
•
8th Grade

Acids Review
•
10th Grade

Molarity & Dilution
•
11th - 12th Grade

Naming Acids and Bases
•
9th - 12th Grade

Acids and Bases
•
9th Grade

Titration
•
10th - 12th Grade