
Introduction to electrolysis
Assessment
•
ROSARIO OLIVARES
•
Chemistry
•
11th Grade
•
54 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
20 questions
Show answers
1.
Multiple Choice
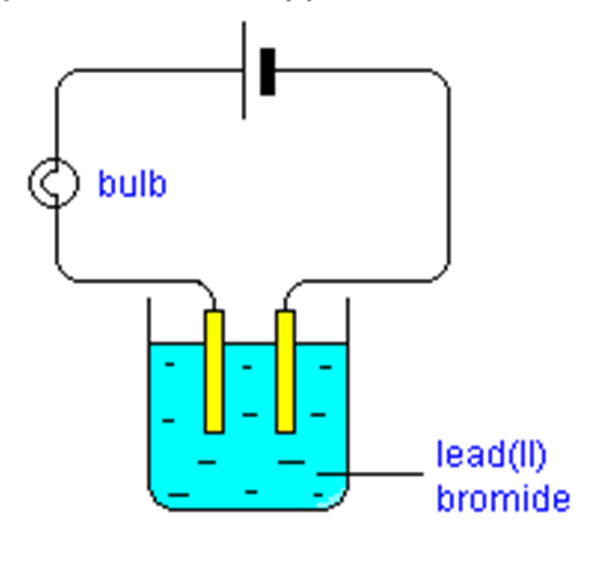
Bromine atoms in lead(II) bromide are converted to ions when it is melted
Electrons flow through the lead(II) bromide when it is melted
The ions in lead(II) bromide are free to move when it is melted
There are no ions in solid lead(II) bromide
2.
Multiple Choice
cathode
anode
3.
Multiple Choice
anode
cathode
4.
Multiple Choice
breaking down of a compound using a current
making a compound using a current
5.
Multiple Choice
The anode is negative and the cathode is positive
The anode and cathode are both positive
The anode is positive and the cathode is negative
The anode and cathode are both negative.
6.
Multiple Choice
Salt solution
Electric solution
Mineral solution
Electrolyte

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Molar Mass
•
10th Grade - University

Moles
•
University

Ionic Compounds
•
9th Grade

Naming Ionic Compounds
•
9th - 11th Grade

Naming Chemical Bond
•
Professional Development

Chemical Reaction and Equation
•
10th Grade

Element Symbols and Names
•
8th - 12th Grade

Ionic Bonding
•
8th - 12th Grade