Entropy - True/False
Assessment
•
Megan Bildner
•
Chemistry
•
12th Grade
•
34 plays
•
Easy
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
12 questions
Show answers
1.
Multiple Choice
Entropy is a form of energy
True
False
2.
Multiple Choice
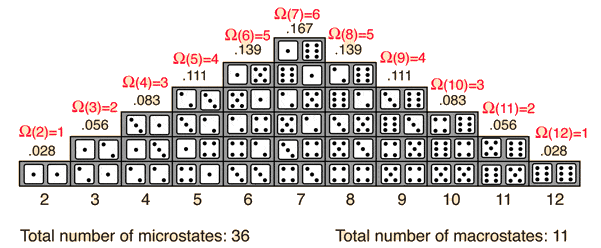
Entropy is a measure of the number of ways in which energy is distributed.
True
False
3.
Multiple Choice
The more widely spread out energy is among available energy levels and particles, the less probable that state.
True
False
4.
Multiple Choice
For a chemical reaction to be feasible, the total entropy change must be zero. (∆Ssystem + ∆Ssurroundings = 0)
True
False
5.
Multiple Choice
Entropy of a system increases when it gains energy.
True
False
6.
Multiple Choice
Entropy of a system increases when it undergoes a change of state.
True
False

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Kinetics
•
9th - 12th Grade

Reaction Rates
•
8th - 11th Grade

Collision Theory and Reaction Rates
•
9th - 11th Grade

Catalysts
•
9th Grade

Basic Kinetics Review
•
10th - 12th Grade

Reaction Rates
•
9th - 12th Grade

Thermodynamics
•
11th - 12th Grade

Introducing Reaction Mechanisms
•
10th - 11th Grade