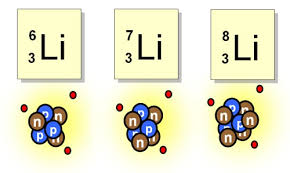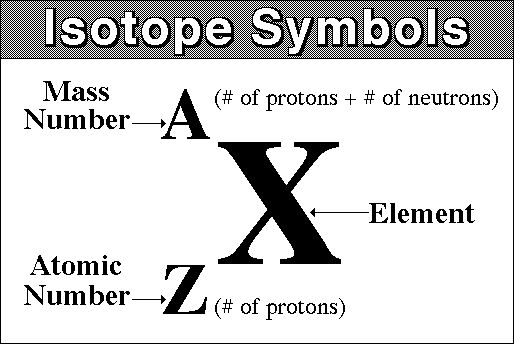
LT3 Isotopic Notation
Assessment
•
ruby disko
•
Chemistry
•
9th - 11th Grade
•
13 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
9 questions
Show answers
1.
Multiple Choice
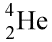
4
2
6
0
2.
Multiple Choice
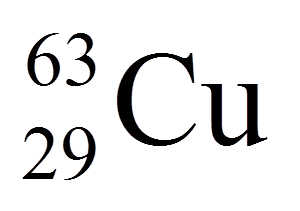
the mass number
the atomic number
the atomic mass
the neutron number
3.
Multiple Choice
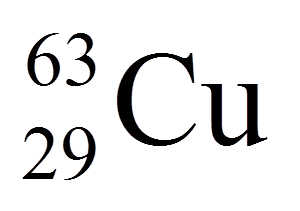
the mass number
the atomic number
the atomic mass
the neutron number
4.
Multiple Choice
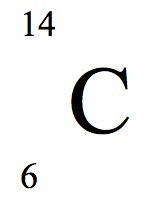
Carbon 12
Carbon 13
Carbon 14
Carbon 15
5.
Multiple Choice
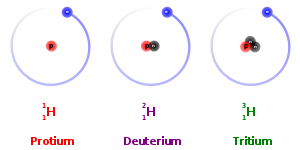
different masses
different atomic numbers
different electrons
different protons
6.
Multiple Choice
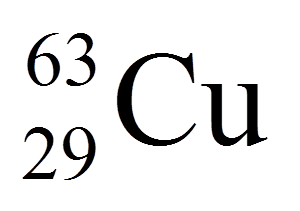
29
34
63
92

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Bohr Models
•
8th - 12th Grade

Introduction to Atoms
•
1st - 7th Grade

Atoms
•
8th Grade

Determining Protons Electrons Neutrons
•
8th Grade

Atomic Structure
•
10th - 11th Grade

Atomic Structure
•
10th Grade

Subatomics Review
•
10th - 11th Grade

Atomic Theory
•
9th Grade