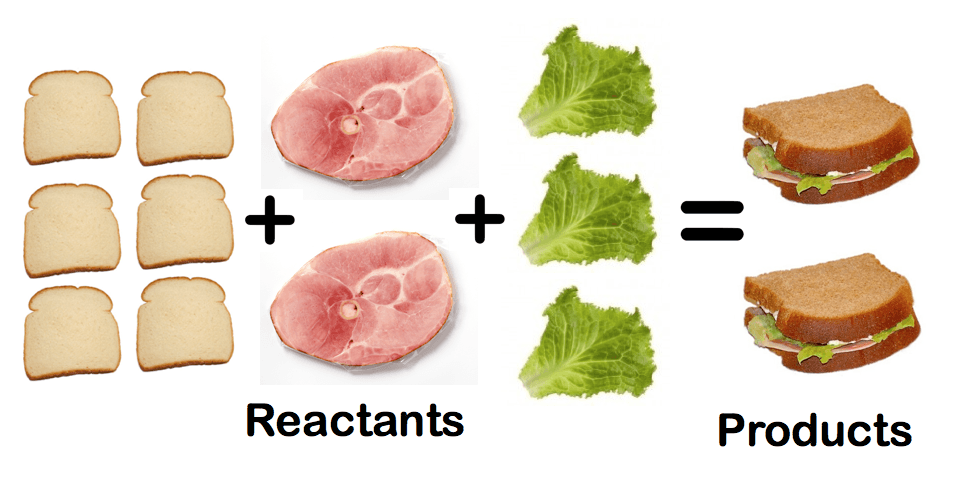
Limiting Reactants & Percent Yield
Assessment
•
Walter Haberaecker
•
Chemistry
•
10th - 11th Grade
•
15 plays
•
Hard
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
10 questions
Show answers
1.
Multiple Choice
slows the reaction down
is used up first
is the reactant that is left over
controls the speed of the reaction
2.
Multiple Choice
Use the equation 2 Al + 3 Cl2 → 2 AlCl3. If 2 moles of aluminum and 2 moles of chlorine are reacted, identify the limiting reactant.
AlCl3
Cl2
Al
3.
Multiple Choice
When the lab is finished
When the excess reactant is used up
When the limiting reactant is used up
Chemical reactions never stop
4.
Multiple Choice
What is the limiting reactant if 10 moles of NH3 react with 30.0 moles of NO?
4NH3+6NO → 5N2 + 6H2O
NH3
NO
N2
water
5.
Multiple Choice
When 12 moles of O2 reacts with 1.1 mole of C10H8 what is the limiting reactant? C10H8 + 12 O2 → 10 CO2 + 4 H2O
Oxygen
C10H8
Water
Carbon Dioxide
6.
Multiple Choice
Theoretical yield = 73 g
Actual yield = 62 g
Calculate the percent yield.
1.16%
116%
85%
76%

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Chemical Equations
•
8th Grade

Types of Reactions
•
10th - 12th Grade

Balancing Chemical Equations
•
8th - 12th Grade

Balancing Equations
•
10th Grade

Classifying Reactions
•
10th - 11th Grade

Types of Chemical Reactions
•
9th - 12th Grade

Chemical Reactions
•
8th Grade

Chemical Equations and Balancing
•
10th - 12th Grade