Unit 3 Review
Assessment
•
Wesley McDade
•
Science
•
8th Grade
•
69 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
36 questions
Show answers
1.
Multiple Choice
What Element has the symbol (Ar)
Arsenic
Astitine
Argon
Gold
2.
Multiple Choice
Does not have a definite volume or shape.
Plasma
Gas
Liquid
Solid
3.
Multiple Choice
Made up of one or more of the same kind of atom chemically combined.
Atom
Compound
Element
Mixture
4.
Multiple Choice
The smallest unit of an element that maintains the properties of that element.
Matter
Law of Conservation of Mass
Atom
Element
5.
Multiple Choice

A sample substance has the chemical formula: H2CO3
One molecule of this sample contains
two different elements with atomic numbers 1 and 27.
two different elements with atomic numbers 2 and 29.
three different elements with atomic numbers 1, 6, and 8.
three different elements with atomic numbers 2, 8, and 20.
6.
Multiple Select
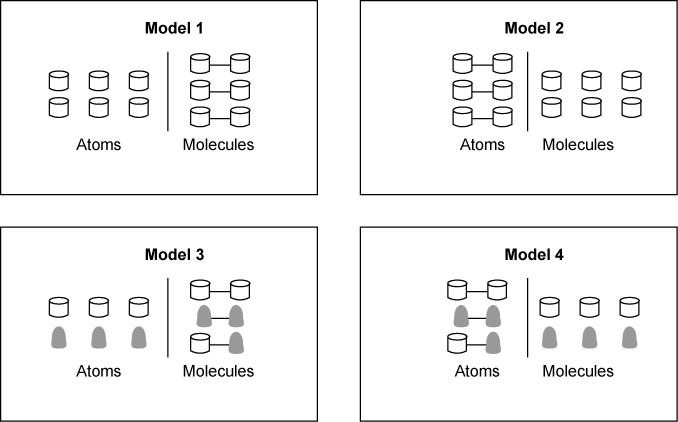
Select two of the conclusions that best describe the diagram.
The substances are matter made from atoms.
The substances are molecules made from atoms.
The substances are elements made from molecules.
The substances are atoms made from elements.
The substances are elements made from matter.

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Atoms and The Periodic Table
•
8th - 10th Grade

Atoms Quiz
•
6th Grade

Atoms and Molecules
•
6th - 8th Grade

Properties of Elements
•
6th - 8th Grade

Periodic Table
•
9th Grade

Atoms
•
5th Grade

Counting Atoms
•
11th - 12th Grade

Atomic Structure
•
8th Grade