The Chemical Basis of Life
Assessment
•
Stacey Zaremba
•
Biology
•
9th Grade
•
200 plays
•
Hard
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
7 questions
Show answers
1.
Multiple Select

Which of these are properties of water?
Water is polar, each molecule has a slight positive/negative charge.
Water is the universal solute.
The hydrogen bonds allow water to stabilize temperatures well.
Water has adhesive properties -- molecules stick to other water molecules.
The hydrogen bonds make water an acid.
2.
Multiple Select
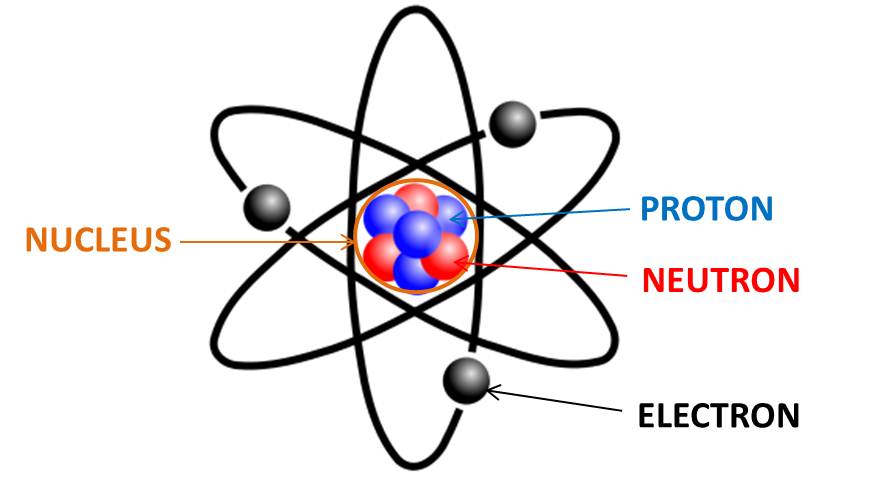
Which of the following are true about atoms?
Uncharged atoms have the same number of protons, electrons, and neutrons.
Atoms of different elements bond together to create compounds.
The atomic number is always equal to the number of electrons.
The atomic mass is the combined number of protons and neutrons.
Sometimes occur as isotopes with more/fewer neutrons.
3.
Multiple Select

Salt...
is formed from a polar covalent bond between sodium and chloride.
contains iodine to help prevent goiters.
is formed when chlorine donates a single electron to sodium.
is an example of an ionic compound between a metal and nonmetal.
4.
Multiple Select
What are the most common elements in the human body?
Carbon
Nitrogen
Water
Oxygen
Hydrogen
5.
Multiple Select
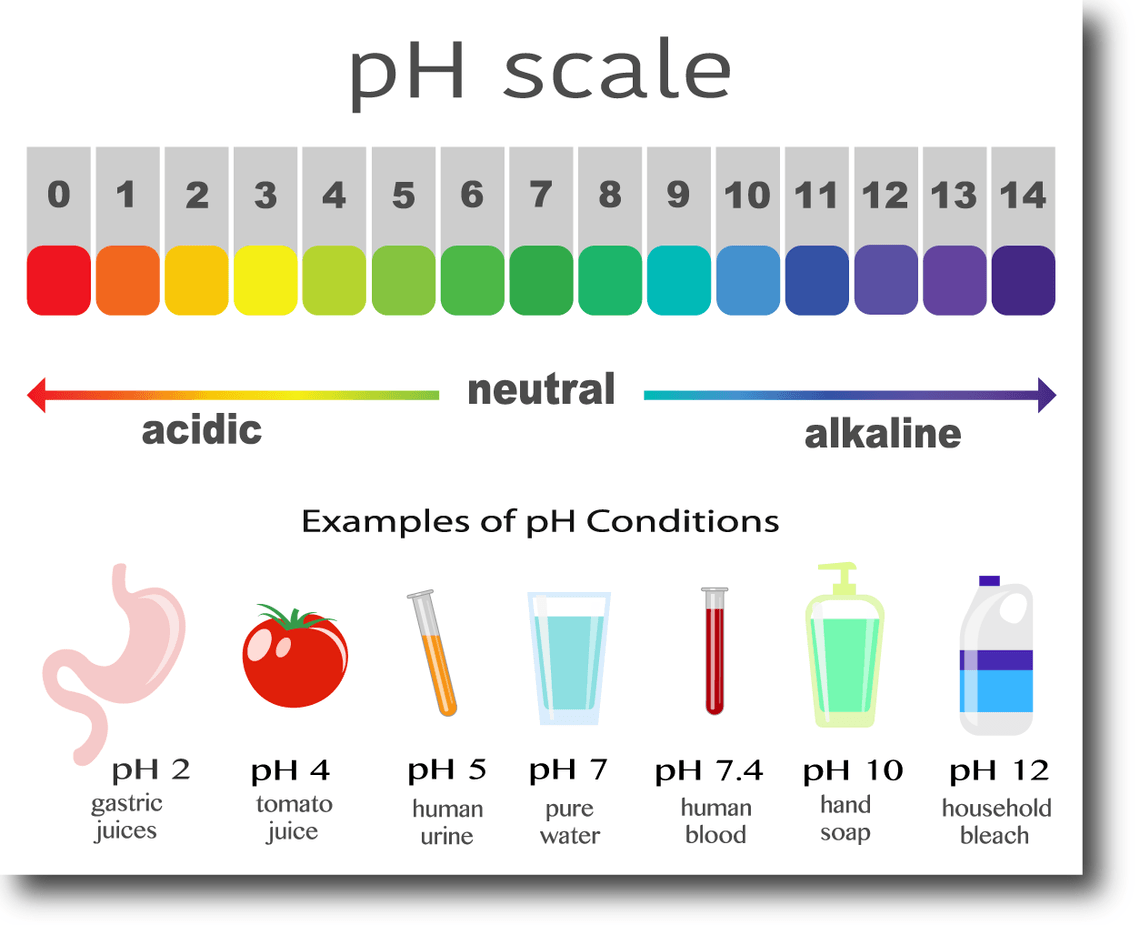
Which of the following are false statements about pH?
A high pH is an indication of an acid.
Acids have an increased number of hydrogen ions present.
Is measured on a scale that progresses by a multiple of 100.
A neutral pH has the same number of OH- ions and H+ ions.
6.
Multiple Select
Hydrogen...
has one electron and one proton.
bonds are responsible for holding nearby molecules together.
atoms in water are slightly positively charged, which are attracted to the slightly negatively charged oxygens of other water molecules.
concentration is measured on a scale called pH
has one valence electron and requires another 7 electrons to fill its outermost orbital.

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Chemistry of Life
•
9th - 12th Grade

Atoms
•
9th Grade

Basic Biochemistry
•
9th - 12th Grade

Atomic Structure and Ions
•
9th - 10th Grade

Evolution
•
4th Grade

Atoms, Elements and Bonds Quiz
•
9th Grade

Atoms Molecules and Ions
•
9th - 12th Grade