Particles of Matter
Assessment
•
Alivia DeLane
•
Science
•
6th Grade
•
54 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
34 questions
Show answers
1.
Multiple Choice
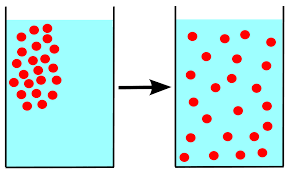
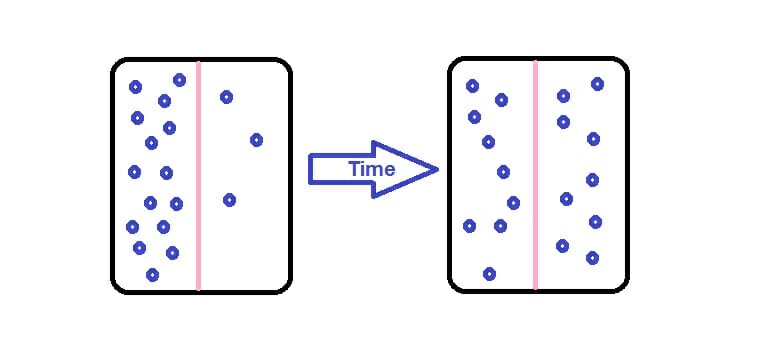
The movement of particles from an area of higher concentration (more particles) to an area of lower concentration. The particles spread out.
Dissolving
Diffusion
Density
Thermal Expansion
2.
Multiple Choice
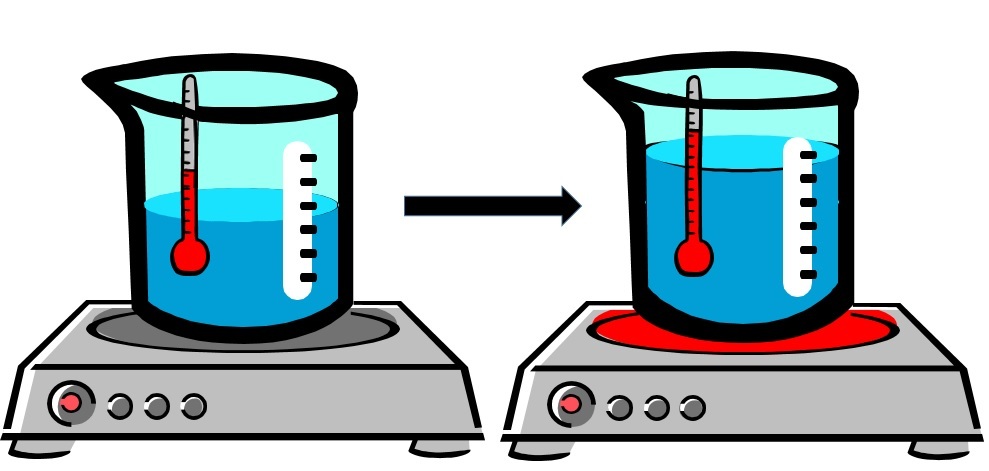
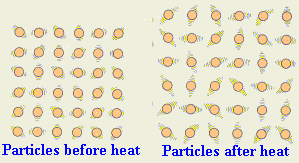
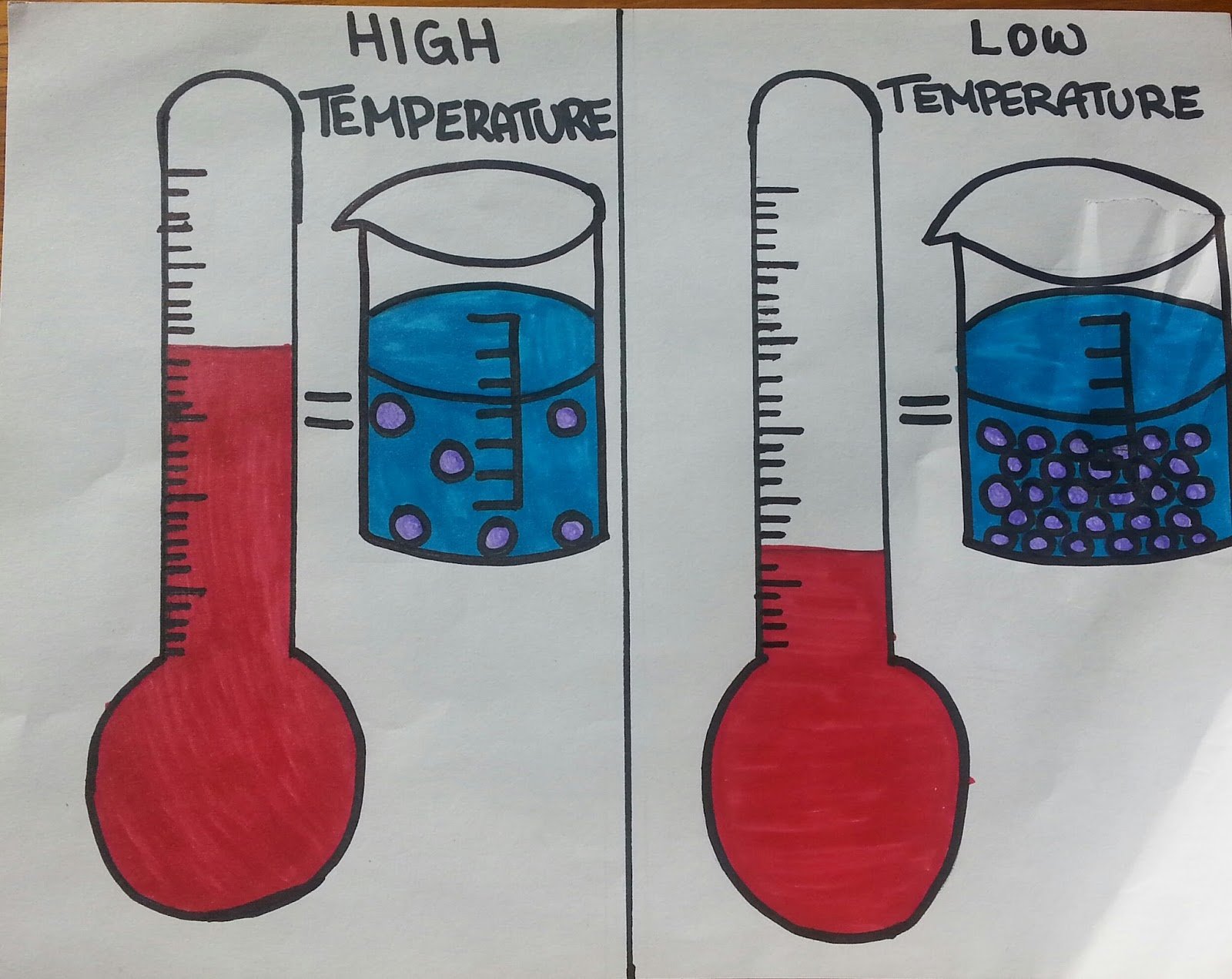
When temperature increases (gets "hotter"), the volume of matter increases (gets bigger).
Dissolving
Compression
Thermal Expansion
Diffusion
3.
Multiple Choice
All _____ is made of tiny _____, called atoms and molecules.
matter, particles
matter, elements
particles, matter
Matter, mixtures
4.
Multiple Choice
The _____, of matter are always _____.
speed, constant
particles, moving
volume, consistent
particles, faster
5.
Multiple Choice
There are always _____ between the particles that make up matter.
movement
spaces
speed
temperature
6.
Multiple Choice
_____ is directly related to the ______ of the particles that make up matter.
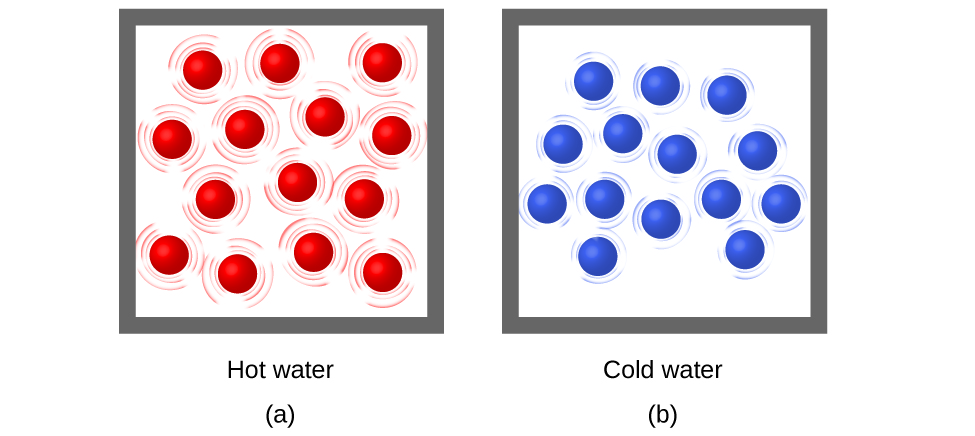
Thermal energy, temperature
Motion, energy
Temperature, speed
Temperature, particles

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Properties of Matter
•
5th Grade

States of Matter
•
8th Grade

Physical Properties of Matter
•
5th Grade

Conservation of Matter
•
3rd - 5th Grade

States of Matter
•
3rd - 4th Grade

States of Matter
•
6th Grade

States of Matter
•
6th - 9th Grade