
Factors affecting Chemical Equilibrium and their Effects
Assessment
•
WONG (WSK)
•
Chemistry
•
10th - 12th Grade
•
85 plays
•
Hard
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
5 questions
Show answers
1.
Multiple Choice
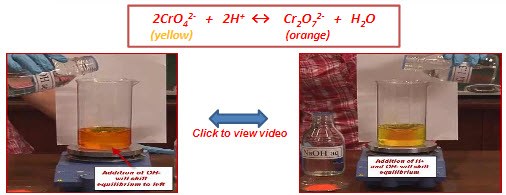
Position of equilibrium will shift to left
Color of system will turn from orange to yellow
Kc will be higher
Equilibrium will shift to right to decrease the added H+ ion
2.
Multiple Choice
1st statement
Increasing reaction temperature can increase the
yield for all reversible chemical reactions.
2nd statement
Increasing reaction temperature can shorten the
time needed to attain equilibrium for all reversible
chemical reactions.
Both statements are true and the 2nd statement is a correct explanation of the 1st statement
Both statements are true but the 2nd statement is NOT a correct
The 1st statement is false but the 2nd statement is true
Both statements are false
3.
Multiple Choice
Refer to the following reversible reaction :
X2(g) + 3Y2(g) <=== > XY3(g)
Which of the following combinations shows the effects of a catalyst on the rate of forward reaction, the rate of backward reaction and the yield of XY3(g) ?
Rate of forward reaction
increased
Rate of backward reaction
increased
Yield of XY3(g)
unchanged
Rate of forward reaction
unchanged
Rate of backward reaction
unchanged
Yield of XY3(g)
unchanged
Rate of forward reaction
increased
Rate of backward reaction
decreased
Yield of XY3(g)
increased
Rate of forward reaction
decreased
Rate of backward reaction
increased
Yield of XY3(g)
decreased
4.
Multiple Choice
The following system attained equilibrium at a certain temperature :
2SO2(g) + O2(g) <===> 2SO3(g)
Which of the following statements is / are correct when the volume of the system is decreased while the temperature remains unchanged ?
(1) The value of KC increases.
(2) The equilibrium position shifts to the right.
(3) The rate of decomposition of SO3(g) increases.
(1) only
(2) only
(1) and (3) only
(2) and (3) only
5.
Multiple Choice
In a closed container and at a certain temperature, the following equilibrium was attained :
COCl2(g) <===> CO(g) + Cl2(g)
Which of the following statements is / are correct ?
(1) CO(g) and Cl2(g) must be of the same concentration.
(2) The rate of decomposition of COCl2(g) is equal to the
rate of formation of CO(g).
(3) The equilibrium constant Kc for the reaction increases
when the volume of the container increases.
(1) only
(2) only
(1) and (3) only
(2) and (3) only

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Types of Reactions
•
10th - 12th Grade

Balancing Equations
•
10th Grade

Balancing Equations
•
10th Grade

Endothermic and Exothermic Reactions
•
9th Grade

Classifying Reactions
•
10th - 11th Grade

Balancing and Types of Reactions Do now
•
6th - 8th Grade

Chemical Reactions
•
8th Grade

Activity Series
•
KG