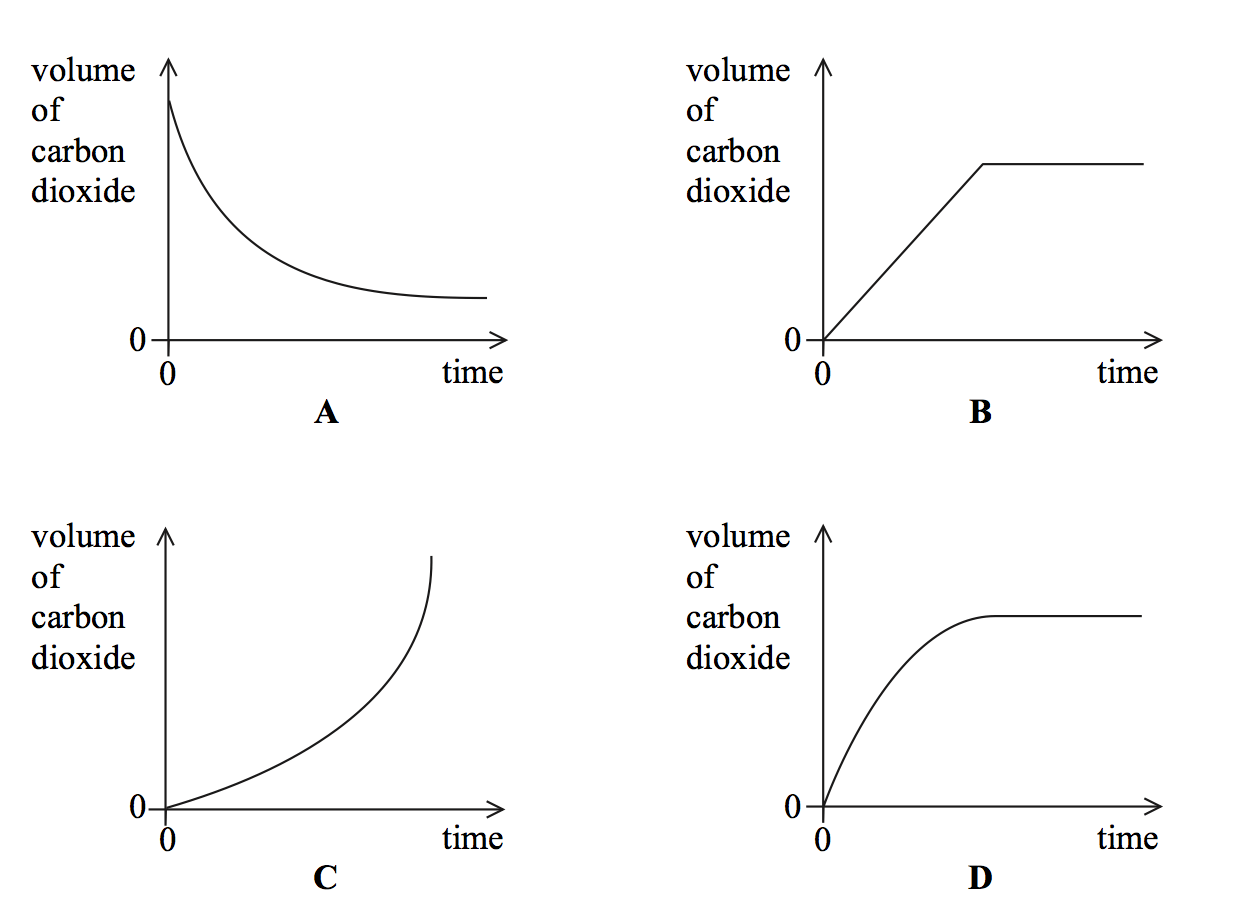Measuring rate of reaction Competition
Assessment
•
Angela Toh
•
Chemistry
•
8th - 10th Grade
•
79 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
30 questions
Show answers
1.
Multiple Choice
How fast a reaction is
How big a reaction is
How loud a reaction is
How much gas a reaction produces
2.
Multiple Choice
Using a gas syringe
Collecting it in a measuring cylinder under water
Measuring the change in mass
Seeing how quickly the solution goes cloudy
3.
Multiple Choice
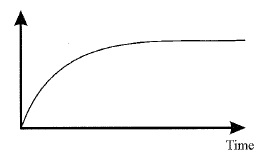
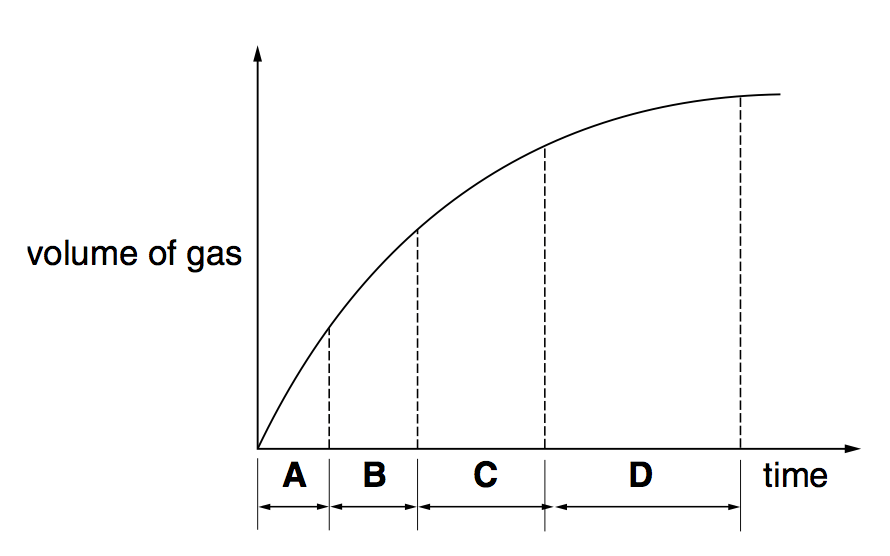
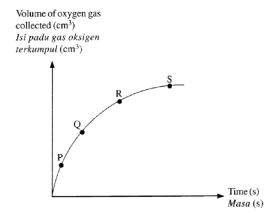
The reaction between zinc and hydrochloric acid may be represented by the equation:
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g);
In an investigation of this reaction using excess Zn and 2 M HCl(aq) in an open flask, the graph shown was plotted from the data collected.
Which of the following would be a suitable quantity for the vertical axis of the graph?
mass of zinc per second
volume of hydrogen gas produced per second
Temperature.
mass loss per second
4.
Multiple Choice

The word for a substance created in a chemical reaction...
Reaction
Reactant
Product
Mass
5.
Multiple Choice
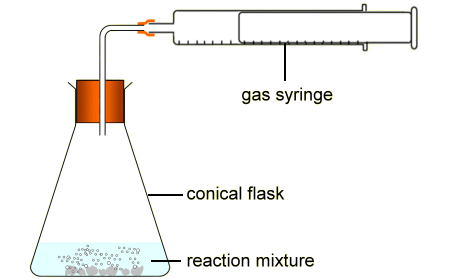
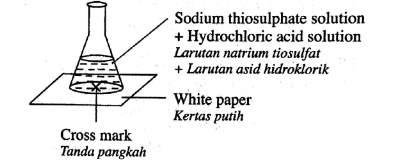
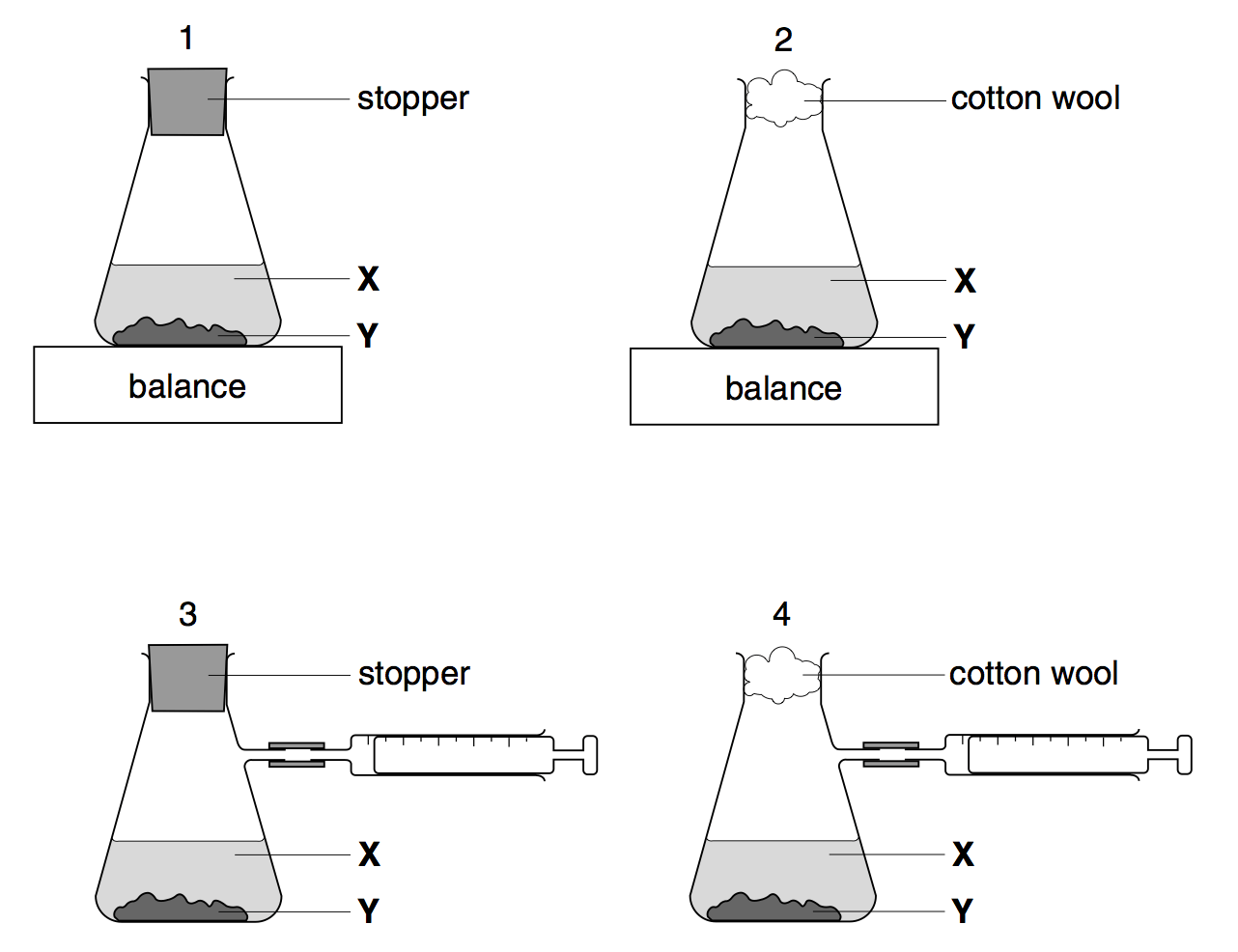
How is this equipment being used to measure the rate of reaction?
The gas syringe measures how much gas is produced in a certain time
The reaction mixture will increase in volume in a certain time
6.
Multiple Choice
If 5cm3 of gas is produced in 20 secs what is the average rate of reaction?
5cm3s-1
0.25cm3s-1
4cm3s-1

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Balancing Equations
•
10th Grade

Energy Changes
•
8th - 12th Grade

Classifying Reactions
•
10th - 11th Grade

Energy Changes
•
10th Grade

Chemical Reactions
•
8th Grade

Haber Process
•
10th Grade

Balancing Chemical Equations
•
KG

Ammonia
•
10th Grade