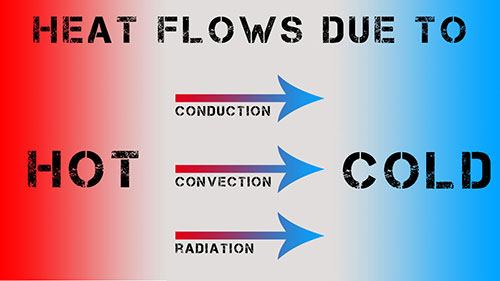
Heat and Temperature
Assessment
•
Christina Cellamare
•
Science
•
6th - 8th Grade
•
1K plays
•
Hard
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
10 questions
Show answers
1.
Multiple Choice
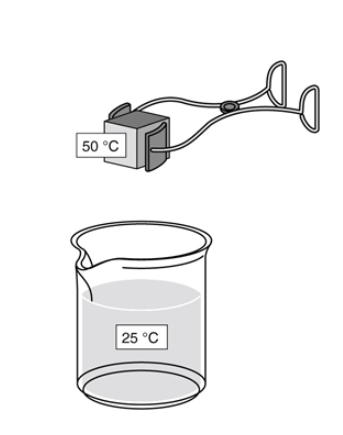
As part of a lab experiment, Tasha drops a metal cube into a beaker of water, as shown in the figure below.
After energy in the form of heat transfers between the substances, what is the final temperature of the water?
The final temperature is 25 °C because there is more water than metal.
The final temperature is 50 °C because the metal warms the water to the temperature of the metal.
The final temperature is between 25 °C and 50 °C because energy is transferred from the metal to the water.
The final temperature is between 25 °C and 50 °C because most of the thermal energy is lost to the air around the substances.
2.
Multiple Choice

The water in Mario’s swimming pool absorbs energy as heat during the day. Which of the following would the water in the pool be most likely to do?
condense
evaporate
freeze
melt
3.
Multiple Choice

During the summer, Chang takes a glass of milk from the refrigerator and places it on the kitchen counter. Over time, energy as heat is transferred to the milk from the air. Which change would most likely occur?
The temperature of the milk will increase.
The temperature of the milk will decrease.
The milk will change from a liquid to a gas.
The milk will change from a liquid to a solid.
4.
Multiple Choice

How is temperature related to heat?
Temperature is a measure of the heat of an object.
Heat causes a change in the temperature of an object.
Raising the temperature causes the heat of an object to increase.
Temperature and heat are two different ways to measure the same thing.
5.
Multiple Choice
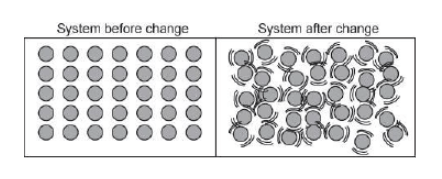
The picture below shows a closed system before and after undergoing a change.
What was changed in the system?
Particles were added to the system.
Water was added to the system.
Heat was added to the system.
Air was added to the system.
6.
Multiple Choice
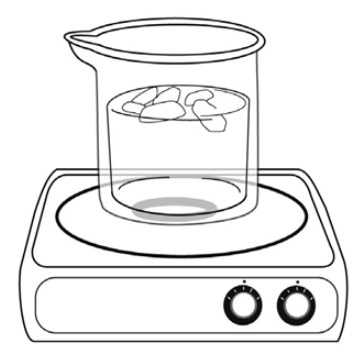
Emerson places a beaker of water on a hotplate and turns the hotplate on. The temperature in the room is 25 ºC. After the water heats to 75 ºC, she adds pieces of ice to the beaker.
Which of the following describes a transfer of heat energy that occurs during this experiment?
from the air to the water
from the ice to the beaker
from the water to the ice
from the water to the hotplate

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Conduction, Convection, Radiation
•
5th Grade

Heat Transfer
•
5th Grade

Thermal Energy and Heat Transfer
•
8th Grade

Conductors & Insulators
•
3rd Grade

Conduction, Convection, & Radiation
•
6th - 8th Grade

Heat Transfer
•
10th Grade

Thermal Energy Transformations
•
6th Grade

Heat Transfer
•
6th Grade