Chemical Bonding
Assessment
•
Crissie Molskness
•
Chemistry
•
9th Grade
•
6K plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
15 questions
Show answers
1.
Multiple Choice
Typically, atoms are more stable when they are
bonded together
apart from each other
2.
Multiple Choice
Why do atoms bond?
They typically don't bond
To add or take away energy levels
To have a full valance shell.
To have a full inner shell
3.
Multiple Choice
Ionic bonding is between a
nonmetal and nonmetal
metal and nonmetal
metal and metal
Depends on the situation
4.
Multiple Choice
Covalent bonding is between a
nonmetal and nonmetal
metal and nonmetal
metal and metal
It depends on the situation
5.
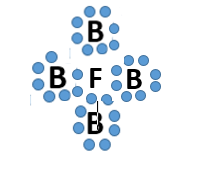
Multiple Choice

Which bond does this picture best represent?
Metallic bond
ionic bond
covalent bond
James Bond
6.
Multiple Choice
What do positive ions tend to do?
lose electrons
gain electrons
lose protons
gain protons

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Types of Chemical Bonds
•
9th - 12th Grade

Covalent Bonding
•
10th - 12th Grade

Lewis Structures
•
10th Grade - University

Bond, Chemical Bond
•
9th - 12th Grade

Chemical Bonds
•
10th Grade

Chemical Bonding
•
10th Grade

Lewis Dot Structures
•
10th - 12th Grade

Covalent Bonding
•
10th - 12th Grade