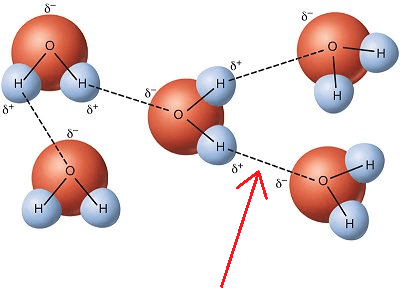
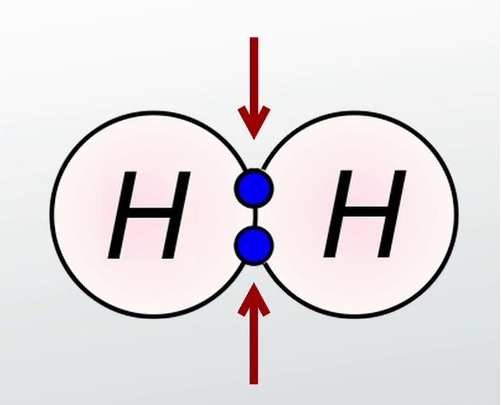
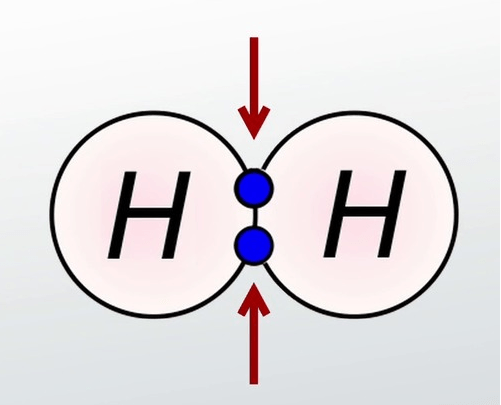
Covalent Bonds
Assessment
•
Jake Apollos
•
Chemistry
•
6th - 10th Grade
•
4K plays
•
Hard
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
10 questions
Show answers
1.
Multiple Choice
Donating & receiving valence e- between atoms.
Opposite slight charges attract each other between compounds.
Scientists are still not sure how they form.
Sharing valence e- between atoms.
2.
Multiple Choice

2 Nonmetals
1 Nonmetal and 1 Metal
2 Metals
2 Noble Gases
3.
Multiple Choice

K2S
H2O
I2
CO2
4.
Multiple Choice
Middle - Each atom shares an octet
Middle - One atom gains an octet & the other loses.
Weakest - it only forms because of another bond forming
Strongest - each atom receives a "permanent" octet.
5.
Multiple Choice
Ionic
Polar Covalent
Nonpolar Covalent
Van der Waals Force
6.
Multiple Choice
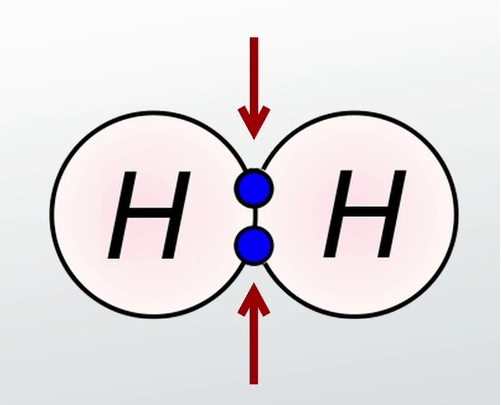
Ionic
Polar Covalent
Nonpolar Covalent
Van der Waals Force

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Types of Chemical Bonds
•
9th - 12th Grade

Covalent Bonding
•
10th - 12th Grade

Lewis Structures
•
10th Grade - University

Bond, Chemical Bond
•
9th - 12th Grade

Chemical Bonds
•
10th Grade

Chemical Bonding
•
10th Grade

Lewis Dot Structures
•
10th - 12th Grade

Covalent Bonding
•
10th - 12th Grade