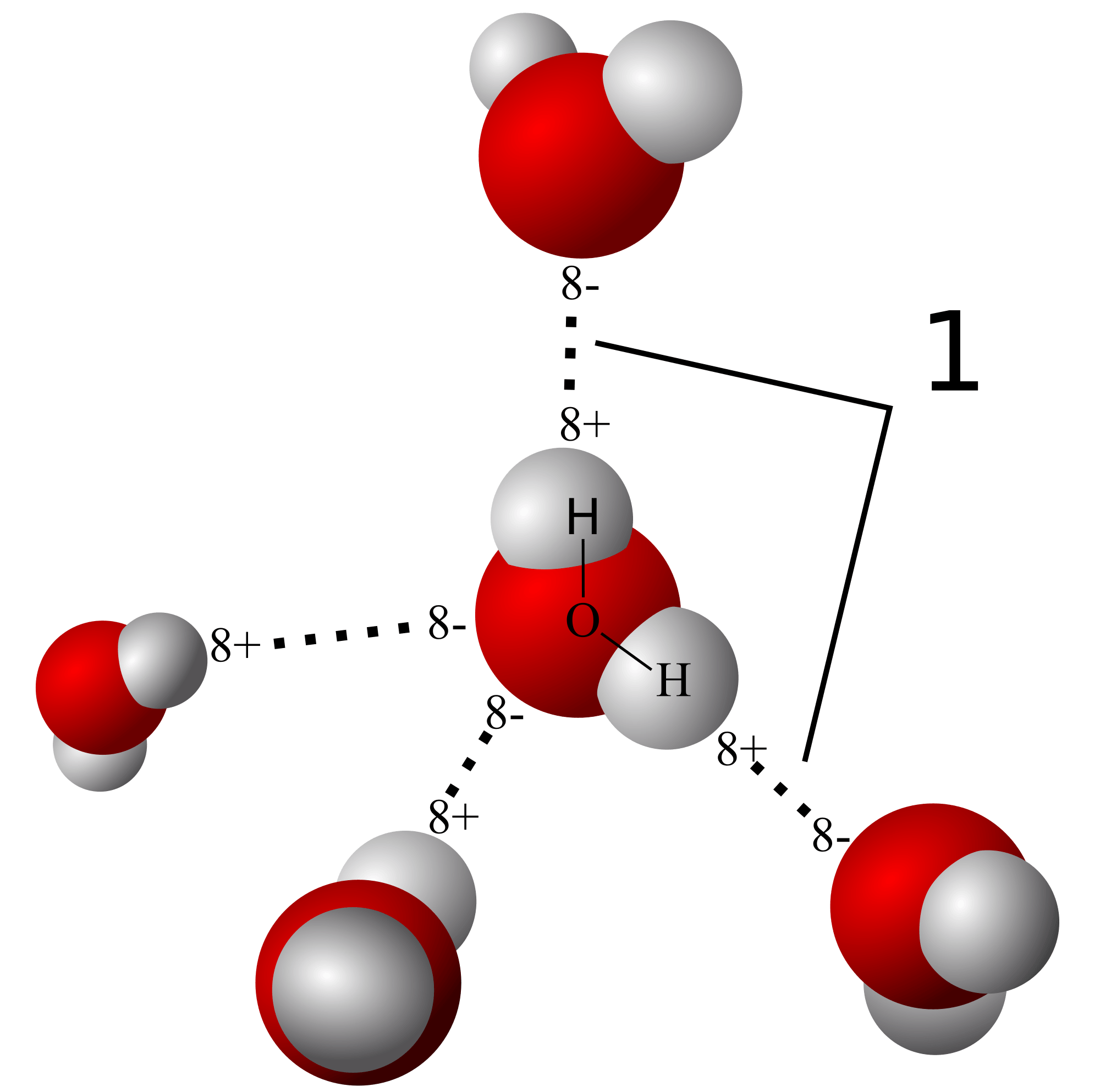
Properties of water
Assessment
•
Deleted User
•
Biology
•
7th - 12th Grade
•
51 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
14 questions
Show answers
1.
Multiple Choice
Nonpolar covalent bonds that enable water to dissolve other substances
Polar covalent bonds that join molecules of water to other substances
Hydrogen bonds between water and another substance
Ionic bonds that enable electrons to flow through water and into another substance
2.
Multiple Choice
Water expands as it freezes.
Water is an excellent solvent.
Water exhibits cohesive behavior.
Water moderates temperature.
3.
Multiple Choice
Purity
Polarity and cohesion
High heat capacity
Expansion upon freezing
4.
Multiple Choice
Water is a solvent.
Water has a high heat capacity.
Water acts as a buffer.
Water is non-polar.
5.
Multiple Choice
The insects are light enough so they do not break the hydrogen bonds holding the water molecules together
The insects actually use their wings to hover slightly above the water’s surface and they only skim it with their feet.
The insect’s feet are non-polar, so they are repelled by the polar water molecules and are pushed away from the water’s surface.
The insects are small enough to see the individual water molecules, so they are able to step carefully from one molecule to the next
6.
Multiple Choice

Helium and oxygen
Hydrogen and oxygen
helium and carbon
oxygen and carbon

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Water, Water Everywhere
•
9th - 12th Grade

Introduction to Ecology & Levels of the Environment
•
6th - 8th Grade

Cell Transport
•
10th Grade

Properties of Water
•
12th Grade

Water Properties
•
9th - 10th Grade

Water Properties
•
9th Grade

Water, Macromolecules and Enzymes
•
9th Grade

Properties of Water
•
9th - 12th Grade