8th Grade Science Review 11 (Periodic Table of Elements)
Assessment
•
Deleted User
•
Chemistry
•
KG - University
•
100 plays
•
Medium
Improve your activity
Higher order questions
Match
•
Reorder
•
Categorization
.svg)
actions
Add similar questions
Add answer explanations
Translate quiz
Tag questions with standards
More options
9 questions
Show answers
1.
Multiple Choice
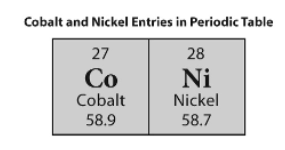
An atom of nickel has more protons.
An atom of cobalt has more electrons.
An atom of cobalt has a lower number of neutrons.
An atom of nickel has a higher value for atomic mass.
2.
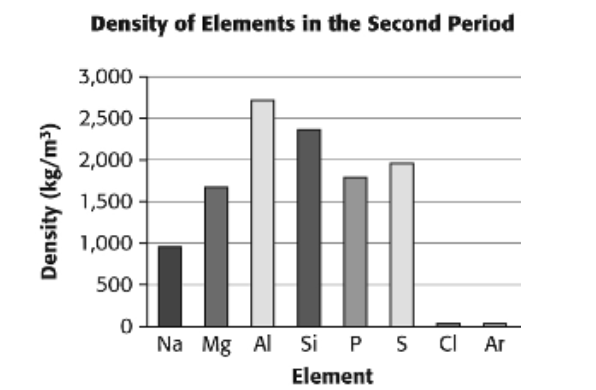
Multiple Choice
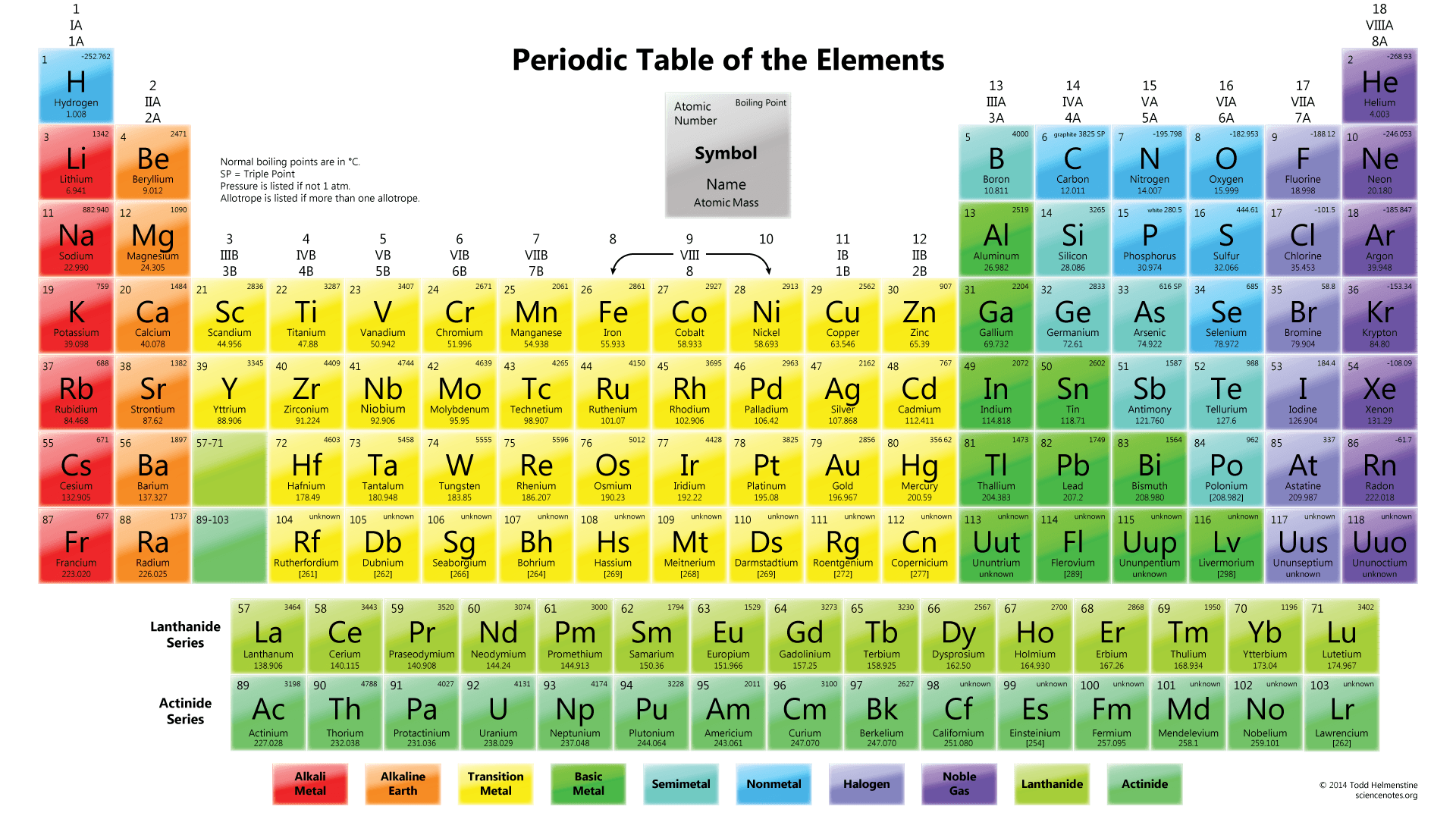
silicon
magnesium
iondine
tungsten
3.
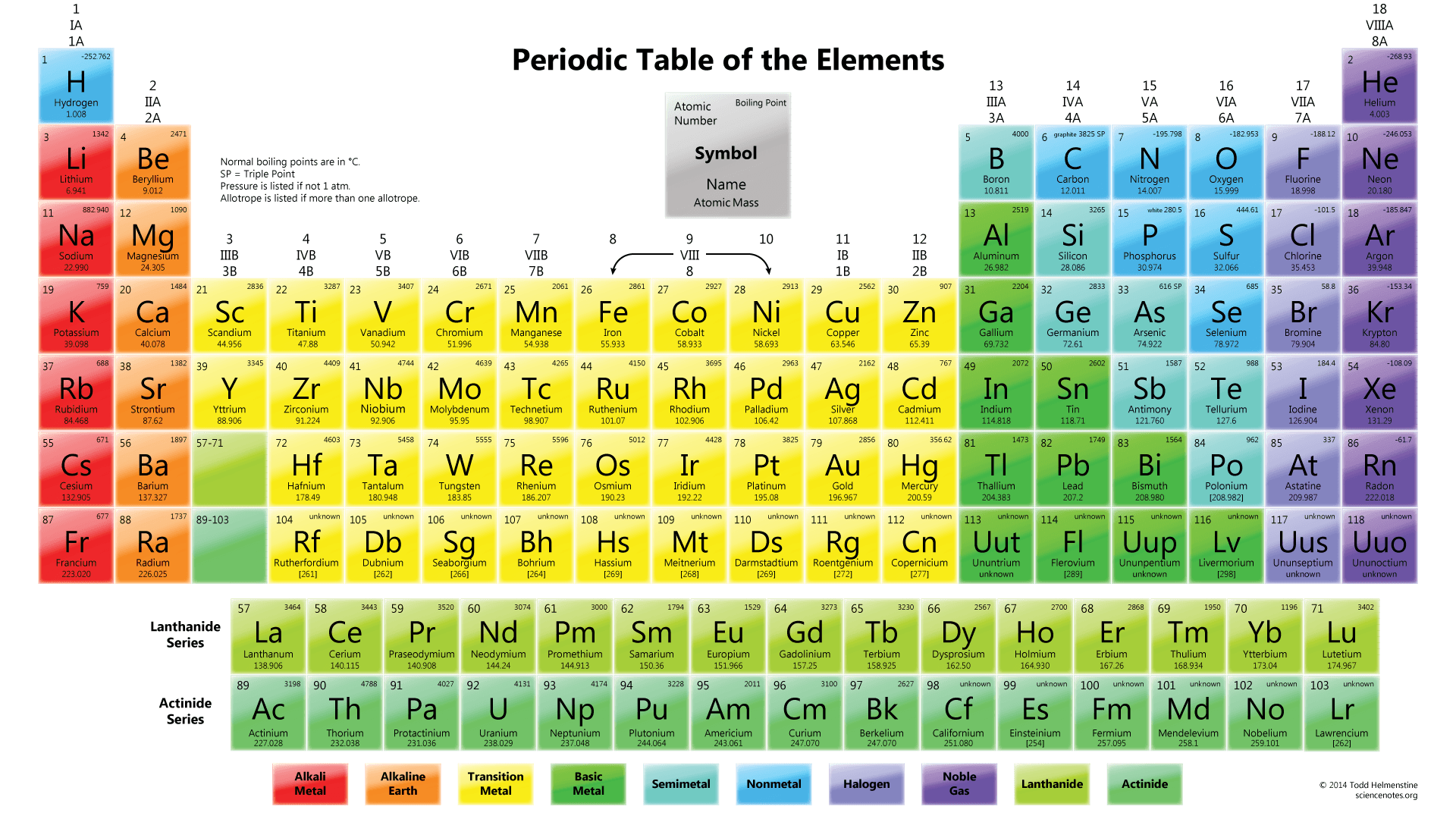
Multiple Choice
inert gases, metals, nonmetals, metalloids
metalloids, metals, nonmetals, inert gases
metals, metalloids, nonmetals, inert gases
nonmetals, inert gases, metals, metalloids
4.
Multiple Choice
They are all very reactive nonmetals with similar chemical properties.
They are all nonreactive gases with similar physical properties.
Their atoms all have eight electrons in their outer energy levels.
They all have the same atomic number.
5.
Multiple Choice
hard, brittle, and nonconductive
liquid, dark, and conductive
shiny, malleable, and conductive
soft, oily, and very reactive
6.
Multiple Choice
by their atomic mass
by their chemical symbol
by their chemical name
by their atomic number

Explore this activity with a free account
Find a similar activity
Create activity tailored to your needs using
.svg)

Periodic Table Trends
•
9th - 12th Grade

Periodic Table Trends
•
10th - 12th Grade

Periodic Trends
•
10th - 11th Grade

Periodic Trends
•
10th Grade

Check Your Periodicity
•
12th Grade - University

Periodic Table Trends
•
8th Grade

Periodic Table
•
8th Grade

Periodic Table
•
9th - 12th Grade